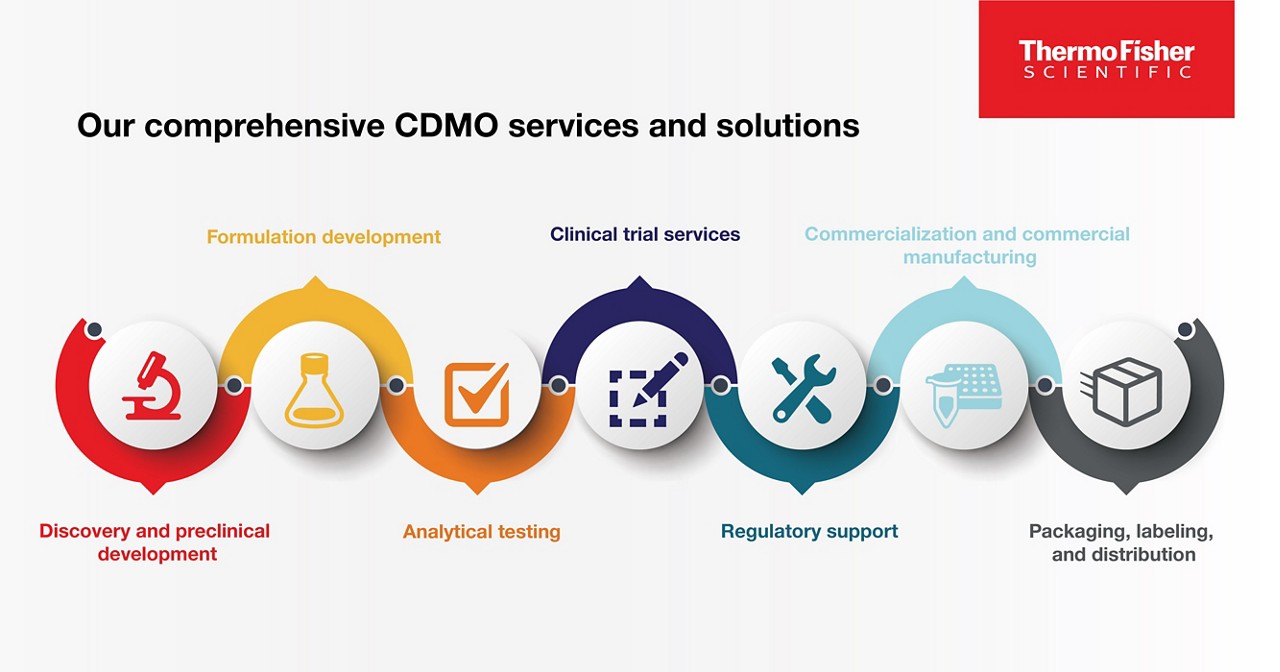
Our comprehensive CDMO services and solutions
Contract development and manufacturing capabilities for small molecules, large molecules, advanced therapies, clinical trials, and commercial services to help move from molecule to medicine to market
The average R&D cost to progress an asset from discovery to launch has remained flat for 2022–2023 at $2.3 billion per asset, including the cost of many failures. Not only is drug development time-consuming and expensive, it also comes with complex challenges from discovery to commercialization — challenges that contract development and manufacturing organizations (CDMOs) can help overcome. Our end-to-end, integrated CDMO capabilities help biotechnology, pharmaceutical, and life sciences companies on their drug development and manufacturing journeys. From research and development to regulatory landscape support, supply chain management, and everywhere in between, our services and solutions support the entire life cycle of drug substances and drug products, from preclinical research to commercial manufacturing.
Whether you have a small molecule, large molecule, or advanced therapy project, our flexible and scalable offerings — including drug development services and drug manufacturing services — enable you to move from molecule to medicine to market with speed and efficiency.
Specifically, our flexible and scalable pharma manufacturing services include:
- Discovery and preclinical development: The early stages of drug development and manufacturing involve research, testing, and evaluation of potential molecules, and it sets the stage for the entire project’s success.
- Formulation development: Formulation development helps optimize a drug’s stability, bioavailability, and efficacy, and ultimately determines the ability of a drug to be commercially manufactured.
- Analytical testing: Evaluating a drug’s formulation development for quality, purity, potency, and safety helps to ensure it meets regulatory requirements and quality standards for patient use..
- Clinical trial services: Our comprehensive clinical trial services include strategy, sourcing, management, packaging and labeling, storage and distribution to help patients around the world.
- Commercialization and commercial manufacturing: It’s one thing to manufacture 100 pills; it’s another to manufacture 1,000,000. Our commercialization and commercial manufacturing can bring your product to market.
- Packaging, labeling, and distribution: Packaging, labeling, and distribution are essential components of the supply chain. Our solutions can help ensure patient safety, product integrity, and regulatory compliance.
- Regulatory support: With around 60 locations across 25 countries, our regulatory experts can help you navigate local landscapes and abide by applicable laws, regulations, and guidelines.
120+ companies
120+ biotech and biopharma companies have partnered with Thermo Fisher Scientific across our CDMO and CRO services on 350+ protocols
>60 locations across 25 countries
Our network of scientists, technicians, and engineers across 25 countries provide unmatched expertise
10-12 billion
Capacity to produce 10 billion to 12 billion softgels annually, backed by a global integrated network to fulfill both large- and small-scale commercial projects
128 approvals
Patheon pharma services has supported 128 regulatory approvals (NDAs/BLAs) during the last five years (2019-2023).
Explore our drug development and manufacturing capabilities
Small molecule
Small molecules come with distinct demands, including solving for solubility and bioavailability challenges, addressing a complex regulatory environment, and ensuring a consistent supply of high-quality raw materials. Regardless of your project’s size or scope, our capabilities for active pharmaceutical ingredients (API), oral solid dose (OSD), steriles, and softgels can support your small molecule’s drug development and manufacturing journey from beginning to end. With our Accelerator™ Drug Development program, you can combine your drug substance and drug product development, demand planning and clinical trial supply execution into a single customized solution to simplify your supply chain and accelerate your discovery through clinical development.
Large molecule
Large molecules, also known as biologics, are complex. Our experience working with 2,000+ molecules — including 230+ oncology molecules — enables us to apply industry-leading expertise to every large molecule project, including yours. Our team can help you transform your discovery into a drug substance via a sustainable process designed to scale from preclinical development to commercial manufacturing. Gain access to our fed-batch, perfusion, and XD® technologies, in addition to our aseptic manufacturing and fill finish solutions for steriles drug development. Plus, our Path to IND for Biologics can help you go from transfection to Investigational New Drug Application (IND) in just 9* months.
Advanced therapies
Advanced therapies — often orphan drugs for rare conditions — require specialized knowledge and the ability to navigate a complex, continuously evolving landscape. We have over two decades of cGMP advanced therapy manufacturing experience, and we’ve manufactured more than 700 viral vector cGMP clinical and commercial lots. Our capabilities for advanced therapies include translational services, clinical supply chain solutions, and the development and manufacturing of viral vectors, cell therapy, and mRNA. With more than 200 advanced therapy clinical trials, more than 150 viral vector products, and FDA- and EMA-approved chimeric antigen receptor (CAR) T-cell therapies, we’re helping biotechnology and pharmaceutical companies revolutionize healthcare.
Clinical trial services
When it comes to clinical trials, biopharma companies face a myriad of challenges. Our end-to-end clinical trial capabilities are powered by people with an unwavering dedication to helping patients around the world. Whether your investigational medicinal product (IMP) is a small molecule, large molecule, or advanced therapy, our 30+ years of in-person, virtual, and decentralized clinical trial experience can help provide support and expertise across your entire supply chain. Our solutions offer the depth, breadth, and flexibility you need to bring your drug to market, including clinical ancillary supplies sourcing and management, label manufacturing, translations, and design, and storage, distribution, and logistics management.
Commercial services
Getting your finalized medicine into the hands of providers and their patients is the final step in the drug development and manufacturing journey, and we can help get you there faster and more efficiently. With industry-leading, global expertise in tech transfer, commercial manufacturing, commercial packaging, and regulatory approvals, we can work with you to achieve parallel active pharmaceutical ingredient (API) and finished dose development — for small and large molecules — delivered on time and on budget. With more than 60 sites across 24 countries, we employ the industry’s best scientists, technicians, and engineers who can help ensure the commercialization of your unique molecule.

