Softgel capsule manufacturing technologies
Designed for agility. Backed by experience. Thermo Fisher proprietary softgel technologies
Whether you’re advancing a new molecule or expanding an established product line, softgels offer a versatile and patient-preferred delivery format. This patient-friendly format is especially effective for delivering poorly water-soluble drugs in lipid-based formulations, making it a strong candidate in early development phases.
Thermo Fisher Scientific brings decades of experience supporting softgel development from early-stage formulation through commercial manufacturing. With dedicated facilities in High Point, NC, and Tilburg, the Netherlands, we have the capacity to produce 10 to 12 billion softgels annually. Our scientists specialize in lipid-based delivery systems and softgel technologies to help overcome complex solubility challenges and accelerate clinical timelines. We also support late-stage lifecycle management and OTC product development to help clients extend product lines and drive commercial value.
10–12 billion softgels produced annually
Robust production capacity backed by 28 encapsulation lines to support programs from development through commercial scale.
25+ years of softgel manufacturing experience
A global network of experts delivering trusted support across every phase of development.
7 proprietary softgel technologies and 60 patents
Innovative, customizable delivery solutions with a strong foundation in scientific and technical IP.
What are the softgel advantages?
- Enhance solubility and bioavailability of poorly water-soluble molecules
- Enable lipid-based delivery for improved absorption and therapeutic efficacy
- Contain highly potent APIs (including Category 3a, 3b, and hormones) with added safety
- Enable precise control of drug release, content uniformity, and absorption site
- Support for taste masking, anti-counterfeiting, and patient-centric design
- Scalable from preclinical development through commercial manufacturing
- Suitable for light- and oxygen-sensitive compounds
- Enable rapid proof-of-concept to gauge market interest
- Support product lifecycle extensions and brand differentiation
- Deliver customized softgel formats with proprietary shell and fill technologies
- Offer solutions for tablet-to-softgel transitions
- Accelerate time to market with streamlined development and regulatory support
- Improve patient experience with enhanced mouthfeel and ease of swallowing
- Enable tailored dissolution profiles to meet specific therapeutic and release requirements
- Support population-specific needs, including pediatric and geriatric applications
Discover the benefits of softgels and our diverse technologies that can meet the needs of your molecules and markets.
Softgel capsule development expertise
Lipid formulations and early development services: A strategic option for solubility challenges:
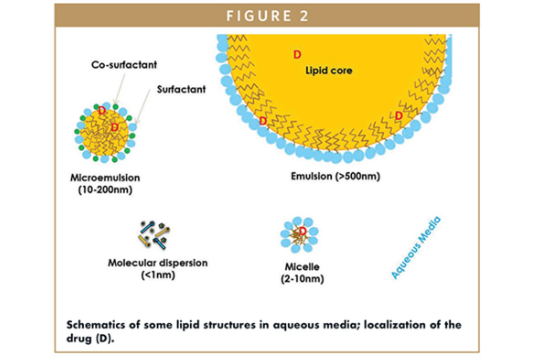
Lipid-based formulations use oils, surfactants, and self-emulsifying systems to deliver poorly water-soluble drugs more effectively. These systems help keep drugs in a dissolved state throughout gastrointestinal transit, improving absorption and therapeutic performance—especially in early development when conventional approaches often fall short.
Our scientists specialize in lipid-based formulations that improve solubility and bioavailability, including self-emulsifying drug delivery systems (SEDDS) and surfactant-based approaches. These systems are ideal for formulating poorly water-soluble molecules, enabling consistent absorption and faster therapeutic onset.
Building on this expertise, Thermo Fisher Scientific supports early-stage softgel development with integrated services that span pre-formulation, analytical development, clinical supply, and scale-up—helping customers de-risk development and accelerate timelines.
Benefits of partnering for early development:
- From formulation to pilot-scale production, all services are coordinated under one global partner.
- Our teams align early with compliance standards to mitigate risk.
- Each formulation is customized based on the molecule’s characteristics and target patient needs.
Over-the-counter (OTC) softgel manufacturing services: Comfort, adherence, versality
Thermo Fisher Scientific also brings decades of experience to the development and commercial manufacturing of over-the-counter softgels. Our integrated capabilities support rapid development, compliance, and scale-up—helping brands bring consumer-preferred formats to market efficiently.
Benefits of partnering for OTC development:
- Comprehensive support from formulation through regulatory approval and commercial production
- Patient-centric solutions that emphasize ease of use, rapid onset, and taste masking
- Global facilities operating under strict cGMP standards and aligned with regulatory expectations
- Agile, large-scale manufacturing to accelerate timelines and adapt to shifting consumer trends
- Proven expertise across a wide range of active ingredients and therapeutic categories
Softgel products and technologies
Thermo Fisher’s 7 proprietary softgel technologies offer flexibility across prescription, OTC, and consumer health products—supporting delivery needs from fast absorption and taste masking to controlled release and pediatric-friendly formats.
Soft lozenges
Our new proprietary soft lozenge technology is uniquely designed to improve patient compliance by providing a soft and soothing sensory mouthfeel, enabling a more comfortable and patient-friendly delivery of active pharmaceutical ingredients (APIs). Soft lozenges are compatible with many different APIs and are suitable for pharmaceutical and nutraceutical products alike.
- Dissolution times can vary from 10-30 minutes
- Less stick, less hard, dissolves in mouth over time
- Superior API taste-masking and shell flavors
- Sugar-free option for specific patient populations
- Suitable for children, seniors, and patients with dysphagia
Chewels®
Chewels® chewable gels are approved for pharmaceutical use and are ideal for pediatric and geriatric populations. They are also well suited for people who find swallowing difficult and are looking for a convenient dosage form for administration.
- Convenient to take without water
- More accurate dosing—less mess
- Lifecycle management solution
- Great for pediatric and geriatric markets
EnteriCare®
EnteriCare® technology incorporates the enteric properties directly into the gelatin shell, replacing extra steps such as coating during the manufacturing process. This creates more consistent enteric behavior. EnteriCare products can be developed as transparent softgels, which creates a more appealing aesthetic quality compared to traditionally coated capsules.
- Enteric properties
- Reduced risk of acid reflux
- No coating
- Targeted delivery
- Proven in existing prescription and nutritional products
Liquisoft™
With a soft-chewable shell, Liquisoft™ softgels are particularly suitable for liquid fills that require a fast onset of action and/or buccal absorption. Liquisoft softgels come in a variety of flavors that mask bad tastes and odors, adding to patient appeal.
- Accurate and convenient dosing
- Soft, chewable shell
- Unique, patented dosage forms*
- Fast-acting for cold and/or cough
*Patents obtained in the United States, Europe, and other countries
Sofgels™
Sofgels™ technology is suitable for liquid formulations, applications requiring faster onset of action, low-dose products, and those with poor bioavailability that would benefit from a lipid system. These capsules are easy to swallow, and twist-off options are available.
- Easier to swallow
- Faster onset of action
- Improved bioavailability
- Accuracy and uniformity in low dosage
- Unique product differentiation
Soflet® Gelcaps
Soflet™ Gelcaps employ a gelatin-enrobing technology that makes tablets easier to swallow. A broad palette of colors and imprinting choices are available, including inline printing.
- Preferred by 3 out of 4 patients*
- Multiple appearance options and palette of colors
- Great for clinical trial blinding
- Could help detain private-label New Biological Entity (NBE) if exclusivity is negotiated
*Based upon study by Banner Life Sciences LLC: Consumer Acceptance of SofletTM Tragon 2002
Versatrol™
Versatrol™ controlled release softgels possess an innovative tamper-resistant technology, making them an excellent choice for abuse-deterrent formulation and/or preventing dose dumping. They are immune to injection, sniffing, crushing, or dissolving. The controlled release properties are never compromised.
- Customized release profile
- Improved bioavailability
- Tamper-resistant technology
- Compatible with lipophilic and hydrophilic formulations
Twist-offs
Twist-offs can help reduce dosing errors observed with other oral liquid formulation by providing the exact amount of liquid needed in the capsule. Twist-offs are suitable for newborns and young infants and are also well-suited for dermatologic products.
- Easily transported for individual dosing
- Prevents recontamination
- Improved bioavailability
- Unique product differentiation
- Accuracy and uniformity in low dosage
Softgel manufacturing sites
Our Tilburg site offers commercial manufacturing and development of gelatin-based softgel drug delivery dosage forms. A Thermo Fisher Scientific Center of Excellence since 1994, the facility provides extensive capabilities across a variety of softgel technologies.
Explore our facility in Tilburg, The Netherlands
Our High Point site focuses on commercial manufacturing and development of softgel dosage forms. Designated as a Center of Excellence since 1996, it offers deep expertise and scale for prescription, OTC, and consumer health softgel products.
Explore our facility in High Point, North Carolina
Helpful resources

