Digital transformation and enablement
Improving the sponsor, patient, and provider experience
The digital transformation known as Pharma 4.0 is revolutionizing process optimization and decision-making within the pharmaceutical industry—at every level and across the clinical development lifecycle and supply chain. At the same time, decentralized clinical trials are now taking a leading role in clinical research. While the approach is not new, the pandemic has increased the incentive for using decentralized trials for future drug development.
We are responding with digital innovations that push the limits of efficiency, agility, and quality for our customers as we adopt networked systems, data analytics, and advanced automation. Customers will see an improved experience at every step. Activities are streamlined, from routine daily status checks or placing orders, to executing a global decentralized clinical trial or manufacturing a full product line.
77%
Digital tools such as mobile technologies, mobile HCPs, and mobile phones are used in 77% of current decentralized trials1
70-90%
Seventy to 90% of molecules in clinical development have bioavailability challenges due to poor solubility
Digital enablement solutions and capabilities
From day-to-day order management and batch tracking to monthly forecasting, our digital mysupply Platform provides timely visibility and real-time collaboration tools to enable more efficient ways of working. mysupply can help you mitigate risk and keep you informed of production status by highlighting where and when your attention is most needed.
- Simple order management: Skip the email and log on to the platform to submit new orders and track existing ones.
- Process transparency: Check the status of your batches throughout the process, including change requests, analytical results, quality deviations, and shipping.
- Automated forecasting: Avoid the manual forecasting and reviewing process by relying on a templated and digital forecast system.
- Streamlined digital experience: Stop digging through emails and files for information. mysupply offers one place to review everything you need.
The platform gives you the ability to view current order status and batches linked to those orders, as well as risks associated with batches and orders. It also allows users to input change requests. Use it to identify delays and proactively address critical alerts and traceability of products.
What’s more, the mysupply Platform provides near real-time data sharing to enhance transparency, build trust, and encourage collaboration among stakeholders.
Forecasts
Automated forecast entry and upload with status tracking to monitor progress.
Orders
Dynamic order submission and tracking capabilities detailing order to batch connection.
Batch tracker
Improved visibility int obatch tracking/status for specific orders.
Dashboard
Up to date, transparent data for collaborative business reviews and daily performance management.
Did you know?
- Remote, virtual, or decentralized trials more than doubled from 2015 to 20192
- 9 out of 10 survey respondents expect COVID-19 to catalyze adoption of deccentralized trial strategies1
Decentralized clinical trials eliminate the need for a patient to visit an investigator site to receive treatment. These trials were on the rise for years before COVID-19, but their success in keeping drug development programs on course during the pandemic won these studies a new leading role in mainstream clinical development and commercialization going forward.
Sponsors are turning to an integrated supply chain solution known as direct-to-patient services to execute decentralized trial strategies. Direct-to-patient services allow patients to participate in clinical trials from their homes by providing them with study drugs and care where they live, using telemedicine and mobile or local healthcare providers.
The direct-to-patient category includes:
Fully decentralized studies, often called virtual or site-less trials. These studies bring the clinical trial to a patient’s home using a central, often virtual, coordinating facility. More than 75% of current decentralized trials use mobile technologies.1 These trials may also harness telehealth, in-home devices, sensors, and wearables, as well as digital therapeutics. The use of artificial intelligence (AI) and machine learning (ML) is also becoming more routine to facilitate diagnoses, patient stratification, and evidence generation.
Hybrid trials incorporate some virtual elements into a site-based study. These studies often access a combination of mobile clinics and patient-reported outcomes.
- Improved patient enrollment and participation
- Shorter trial timelines
- More diverse/representative patient populations
- Reduced cost
- Real-time data
- Easier patient recruitment
- No need to store study drugs and ancillaries during the duration of a study
- Less administrative burden on site staff
- Reduced time and travel burden, making easier to recruit and retain patients
Helpful resources
Quadrant 2
Predicting solubility and bioavailability enhancement
Our proprietary computational modeling program, Quadrant 2TM , analyzes your compound’s specific molecular structure and chemical characteristics, in combination with your unique target product profile. The platform is built on exclusive algorithms that integrate a range of computational methods, including quantum mechanics, molecular dynamics, quantitative structure-activity relationship (QSAR) modeling, ADMET (Absorption, Distribution, Metabolism, Elimination, Toxicity) studies, statistical analysis, and internally developed models.
Quadrant 2 analyzes the molecular structure and the physical and chemical characteristics of a compound based on the following information:
- Active pharmaceutical ingredient (API) chemical structure
- Physicochemical properties
- Full-scale molecular modeling based on quantum and molecular dynamics simulations
- Exclusive excipient descriptor database developed by Patheon pharma services
With the individualized data of a compound, Quadrant 2’s predictive modeling capabilities allow it to:
- Identify the most suitable solubility enhancement technology and excipient combination, aligning with the clinical and business objectives of the development stage
- Discard solubility enhancement options that are less likely to be beneficial, significantly reducing the time and investment typically lost in trial-and-error experimentation
Quadrant 2 accurately identifies technologies for solubility enhancement with a 90% success rate and selects excipients with an 80% accuracy rate. Using the data provided through the Quadrant 2 computational model conserves valuable API in the early stages of development.
Helpful resources
Browse our resource library to learn more about Quadrant 2 and other digital modeling capabilties.
Pharma 4.0 is a new operating model for pharmaceutical factories and supply chains of the future, including new digitized and automated manufacturing approaches.
We are aligning across our network using processes that are more agile, collaborative and technology driven. The three primary ways we are doing that are as follows: Manufacturing technology and automation, including standardized processes and improved capacity; creating a digital shop floor and factory of the future; and implementing a digital toolbox and optimized processes to improve results.
Pharma 4.0 benefits to customers include:
- Faster decision making
- Real-time control of operations and quality
- Global capacity expansion
- Significant productivity improvements
- Data integrity and transparency
- Seamless compliance
- Reduced deviations
- Time and money savings
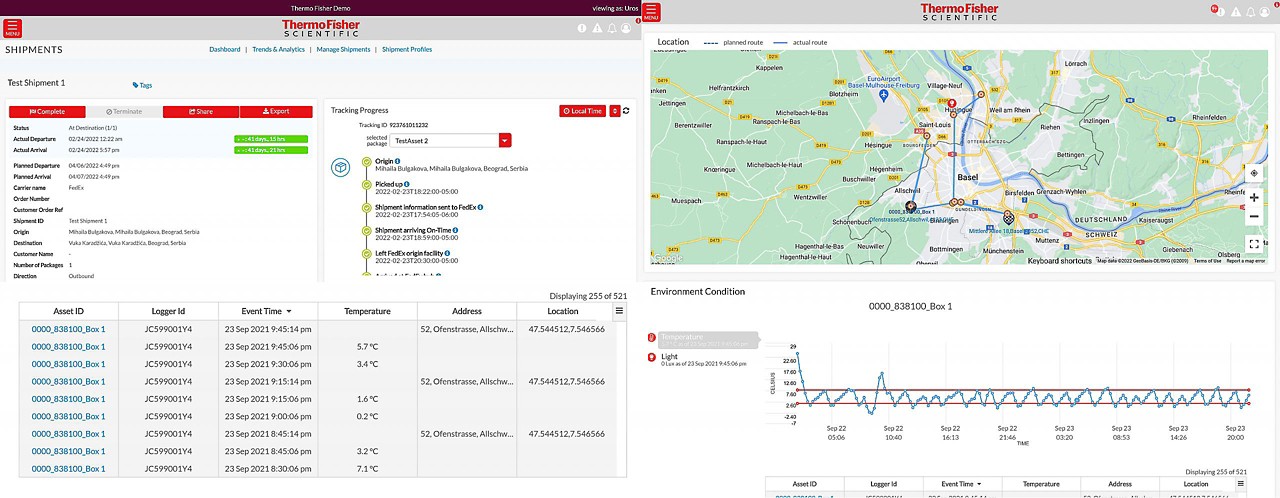
Our Real-time Track and Trace solution enables our customers to make the world healthier, cleaner, and safer through enhanced shipment visibility.
Global visibility anywhere, anytime.
- Track all your shipments in one place
- Stay informed every step of the way
- View your cold chain shipments using real-time temperature and location tracking
- Track delays and excursions
- Advanced monitoring for critical cold chain and high-value product shipments
Track and Trace digital portal
Track all your shipments in one place.
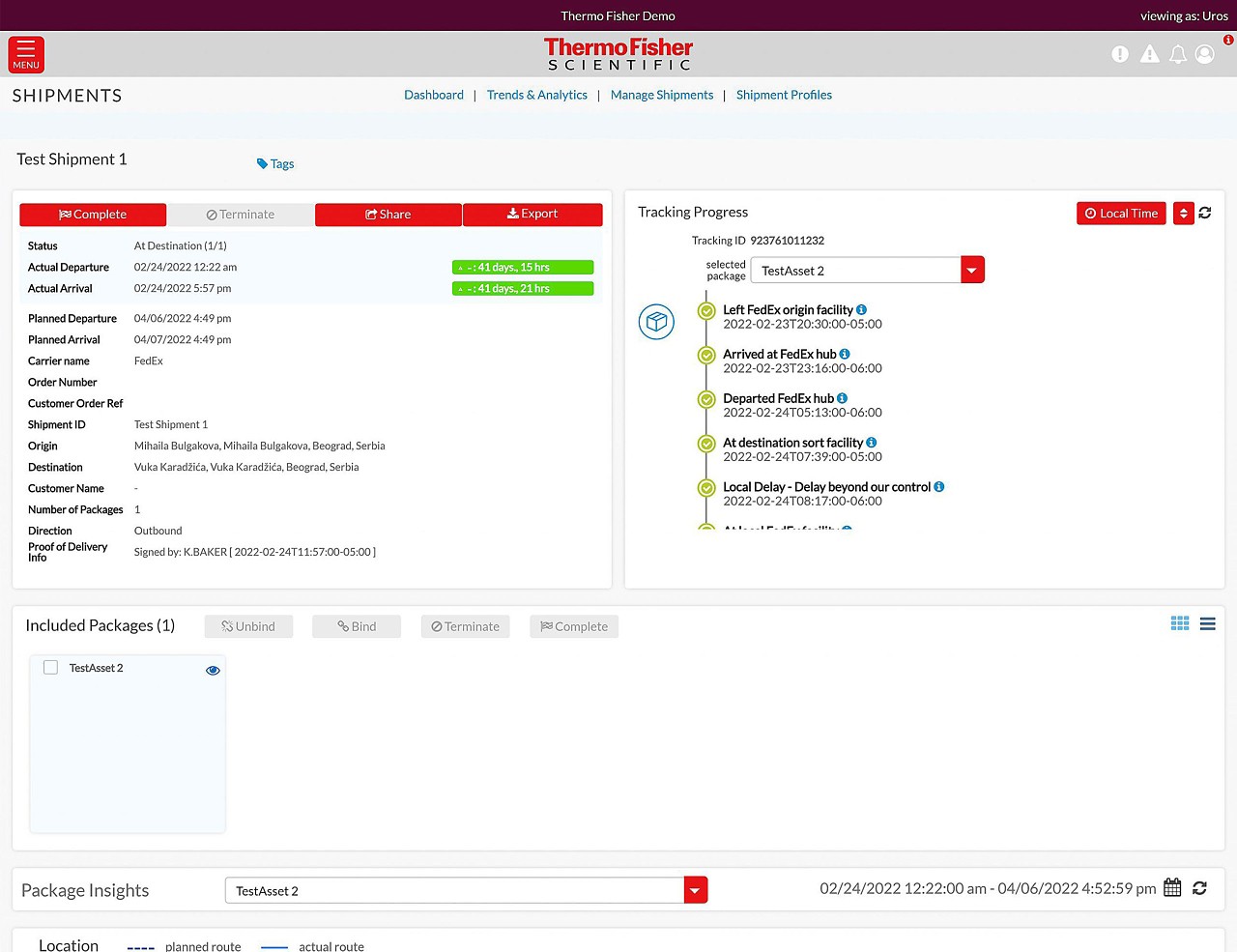
Real-time sensors
View your cold chain shipments using real-time temperature and location tracking.
Smart packaging uses microcircuits to passively measure dosing data and connects to the cloud via technology such as the patient’s smartphone. Cloud-based platforms then utilize sophisticated algorithms to analyze patient dosing behaviors and identify irregularities, enabling site staff to intervene and help patients adhere to their prescribed regimen.
Smart packaging for medication adherence provides a rich and reliable data set, enhancing the understanding of patient dosing patterns, which makes it applicable to most clinical trial designs. It is particularly beneficial for clinical trials that:
- Require precise tracking of the intervals between doses
- Have complex dosing regimens
- Last longer than one year
- Include long intervals between visits to clinical sites (decentralized clinical trials)
This state-of-the-art facility serves as a coworking lab space to pilot and showcase our future-thinking services and innovative solutions for the global clinical trials industry.
The facility in Center Valley, PA supports four key functions, including:
- Create, test, and validate new services and products (working lab)
- Partner and innovate with our clients and colleagues (co-creation)
- Showcase existing innovative capabilities and new services
- Internal/external training space for employees and colleagues

Clinical trials require robust setup and planning processes to ensure the validity of the study outcomes. Rigorous setup and planning help to define clear protocols, select appropriate participants, and establish reliable methodologies — ultimately contributing to the integrity of the clinical trial’s results.
Thermo Fisher Scientific’s Trial Setup and Planning (TSP) system is a comprehensive solution designed to optimize your study while empowering you to achieve greater efficiency, accuracy, and success in your clinical trial planning and execution.
Some key benefits that our TSP solution provides include:
- Robust demand planning and supply coordination
- Strategic study design and supply chain optimization
- Access to clinical trial expertise and support
- Real-time visibility into study dynamics
In silico modeling technologies are transforming drug development and manufacturing by enhancing efficiency and accuracy, enabling innovation. Powered by artificial intelligence, machine learning, bioinformatics, molecular dynamics simulations, and systems biology, these tools accelerate the development of new drugs, optimize formulations, ensure manufacturing quality, and streamline regulatory processes, all while potentially reducing costs and time to market.
Thermo Fisher Scientific's advanced computational and digital modeling capabilities, collectively known as Engineered Solutions, support a comprehensive approach to drug product development by enabling smarter decisions through a deeper understanding of different product parameters.
Utilizing both proprietary and industry-standard technologies, these solutions are key in de-risking and accelerating every phase of drug development. Specific applications include:
- AI and machine learning-enabled predictive modeling for solubility and bioavailability enhancement
- Accelerated stability modeling for shelf life and packaging determination
- Materials science, compaction simulation, and process modeling
- ADME-PK modeling to predict the effect of API physiochemical traits on its pharmacokinetics
Advantages of Thermo Fisher Scientific’s Engineered Solutions:
- Mitigate development risks and reduce experimental failures
- Accelerate development timelines
- Lower overall costs
- Conserve valuable API resources
- Empower informed decision-making and rational drug design
- Reduce reliance on animal testing
View references

