mRNA development and cGMP manufacturing services
Innovative and flexible end-to-end solutions for mRNA workflow under one roof
The rapid development and approval of mRNA-based vaccines during the COVID-19 pandemic spurred a renewed interest in mRNA technology, creating market constraints on access to critical raw materials and manufacturing capacity. Thermo Fisher Scientific responded quickly by ramping up a flexible solutions model for mRNA vaccines and therapeutic applications that spans the entire operational value chain, from DNA template generation to cold chain logistics. Save time and costs with our end-to-end solutions that provide support from early clinical phases through commercialization—all located under one roof at our center of excellence in Monza, Italy.
A legacy of innovation and technical expertise
We bring 30+ years of sterile fill-finish, biologics, and advanced therapy manufacturing experience, with a proven track record of bringing molecules from concept to commercialization. Our Monza center of excellence for sterile fill-finish and mRNA services has a strong regulatory track record, with 80+ successful regulatory inspections across 13 different health authorities since 2004. This legacy of technical expertise, production experience, and robust global quality systems is leveraged in our latest mRNA service offering, combined with ongoing in-house innovations to help you optimize product performance and accelerate time to market.
Flexible scale options for your unique mRNA vaccine or therapeutic needs
From global vaccines to personalized cancer therapies, mRNA is being considered for a variety of clinical applications, creating varying requirements for production volume and quality. To address this evolving demand, we provide flexible options in both scale and quality to support small- and large-volume projects from <1 g to 100 g for mRNA and other RNA modalities (self-amplifying, circular, etc.). Additionally, we can integrate your existing mRNA processes or help you in leveraging the in-house processes developed at our Monza site.
À la carte options within an integrated mRNA CDMO service offering
By combining a flexible approach to development and manufacturing with critical foundational capabilities like comprehensive analytics and regulatory support, we provide the speed and expertise needed to succeed in today’s rapidly evolving mRNA market. From process development to cGMP production, choose from our core mRNA service options and add upstream and downstream solutions as needed. We also offer access to internally developed assets that can fit seamlessly into your workflow, helping to save both time and costs. This includes our license-free, customizable pTRXi plasmid DNA backbone, which supports a range of GOI sizes. When partnering with Thermo Fisher, you have the option to leverage our full suite of integrated services and solutions or choose those that help address gaps in your capabilities or capacity.
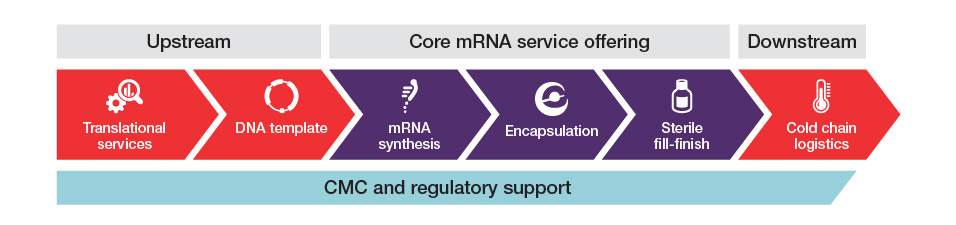
Our team employs a methodical approach to ensure clinical and commercial readiness, focusing on process and analytical method optimization, followed by thorough verification and confirmation. This strategy helps to mitigate risk, ultimately saving time and reducing costs. Key elements of our mRNA process and analytical development service are described below:
- Process characterization studies
- Scale-up and optimization (μL to L scale)
- mRNA synthesis workflow definition and optimization
- Lipid formulation and solvent removal development
- Fill-finish services, such as formulation and lyophilization recipe development and process characterization studies
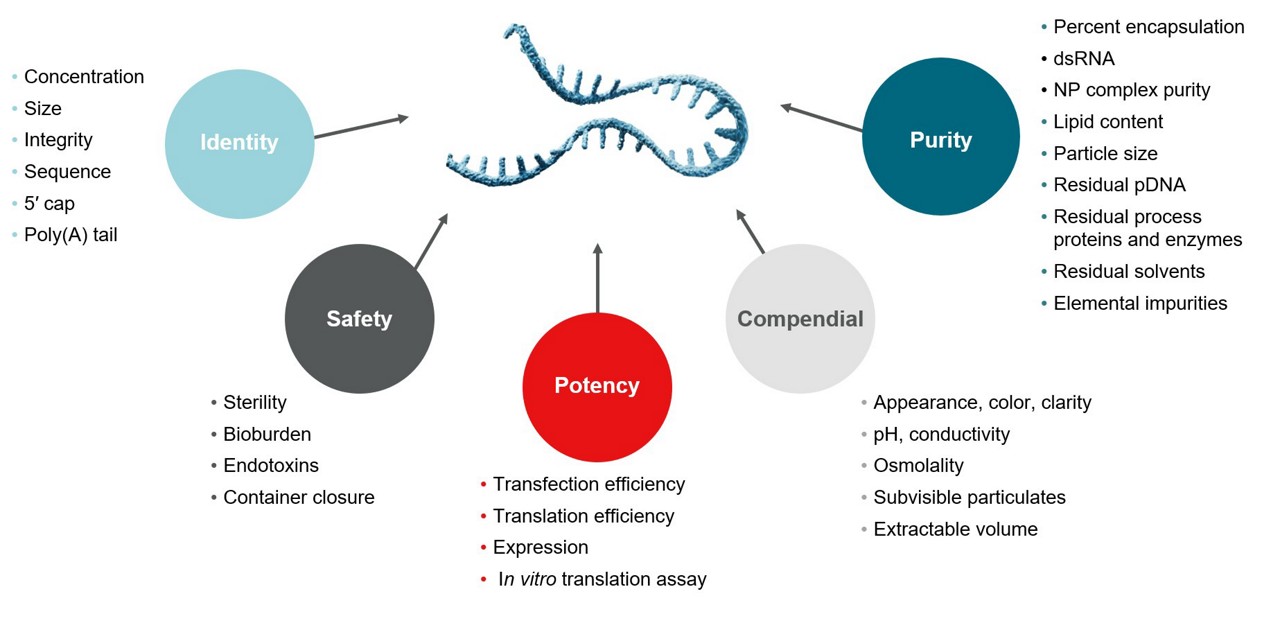
- Development and qualification of analytical methods to ensure the quality and effectiveness of RNA and LNP products leveraging industry-leading methods such as NGS, HPLC, and more.
mRNA-LNP process development |
Compatibility study |
Familiarization study |
Raw material evaluation
|
Robustness study |
Gap assessment |
mRNA-LNP purification |
Technology transfer |
mRNA-LNP stability studies |
Follow-up |
- Three process trains for RNA synthesis and purification; up to over 100 g mRNA/batch scale
- Two lipid nanoparticle formulation areas; 500 L working volumes
- Up to five modular suites for synthesis and purification
- Up to four modular lipid nanoparticle formulation areas
- Ability to support from early clinical to commercial (from <1 g to 100 g scale)
- Max capacity 15 kg/year
- Dedicated HVAC and utilities for suites to prevent cross-contamination
- Production areas maintained to ISO 7/Grade C

View our infographic for an overview of a standard mRNA manufacturing workflow and insights on how Thermo Fisher Scientific can support each step.
Our team can provide various lipid nanoparticle (LNP) manufacturing solutions using either microfluidic or t-mixing technologies, including comprehensive process and analytical development tools. To provide flexibility for our customers, we are open to various lipid technologies. This can involve access to proprietary lipids from within the Thermo Fisher network or optimizing a client's existing lipid strategy using a systematic DOE-based approach (design of experiments).
We are also well-equipped to handle technology transfer projects. Regardless of the approach, we work closely with our clients to develop tailored solutions to seamlessly integrate their LNP workflow into our facilities, identifying any gaps and proposing process optimizations as needed.
Our 642,000 sq. ft. campus in Monza, Italy, is a center of excellence for prefilled syringe and cartridge manufacturing, as well as mRNA drug substances. This unique co-location of mRNA manufacturing capabilities with LNP and fill-finish services helps to streamline your development journey and mitigate risk on your way to market.
Helpful Resources