Sterile fill-finish services
Efficiently complete the manufacturing process for your parenteral, injectable medicines
As a proven leader in the production of biologics and small molecules from first-in-human (FIH) trials to commercial-scale manufacturing, we can help you advance to market quicker. With many format and formulation choices available including lyophilized, we can manufacture the right format for every stage of the product lifecycle.
Flexible sterile fill-finish services at our cGMP facilities can accommodate a variety of molecules including biologics and mRNA. For preclinical work, non-cGMP options are also available. Our global teams and regulatory experts have guided numerous projects to successful NDA/BLA approval — we can do the same for you.
Vial, prefilled syringe, and cartridge fill-finish offerings
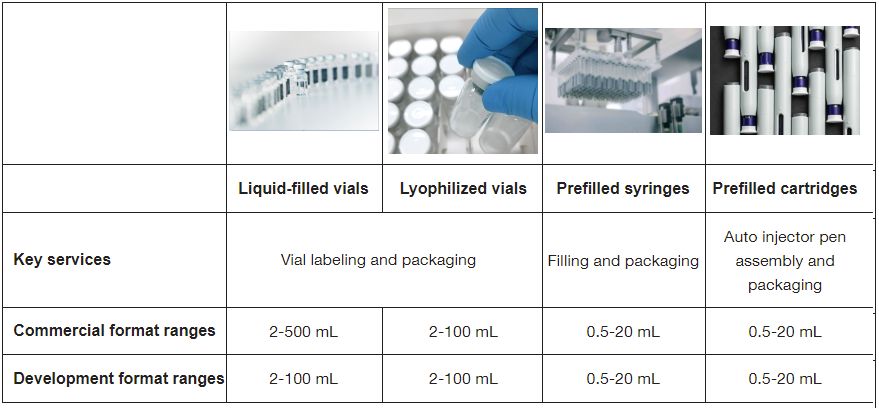
We offer a broad range of sterile parenteral dosage formats to meet your molecule’s specific needs as you move through preclinical to first-in-human (FIH) studies and beyond.
 |
 |
 |
 |
|
| Key services | ||||
| Commercial format ranges | ||||
| Development format ranges | ||||
Manufacturing sites are global including US and EU. Contact your representative for site-specific information.
Aseptic fill-finish solutions for parenteral drug products
Work with a leading CDMO of parenteral/injectables to take your medicine from formulation through commercialization. Our teams can help at every phase of the product lifecycle, leveraging decades of experience to help you produce different parenteral drug formulations as your needs evolve.
Developing the correct formulation can be time-consuming when you can least afford delays. The chemical nature of the compound may present significant challenges like temperature and shear sensitivity, poor solubility, and high potency. Lyophilization, if desired, requires cycle optimization.
Our established parenteral formulation processes include:
1. Pre-formulation: Optimal pH, ionic strength, degradation pathways, and stability studies
2. Formulation development: Select and define optimal levels for all excipients using OFaT and/or design of experiments (DoE) approaches
3. Formulation confirmation: Confirm stability of the selected final formulation using analytical tools
Learn more about our formulation services for:

Reduce costs and assure quality during preclinical and phase I studies
Preclinical and phase I studies can have lengthy timelines that lead to increased costs. Ensuring quality and consistency across processes in early development is critical as you move from phase to phase. Lyophilization, use of non-cGMP batches, and pre-qualified components can address both concerns. Lyophilization increases stability, leading to a longer shelf-life for the duration of clinical trials.
To save time and money, we offer non-cGMP suites that ensure scalable equipment. The setup of our non-cGMP lines mimics our cGMP lines — it’s the same material without the aseptic processing and costs associated with cGMP spaces.
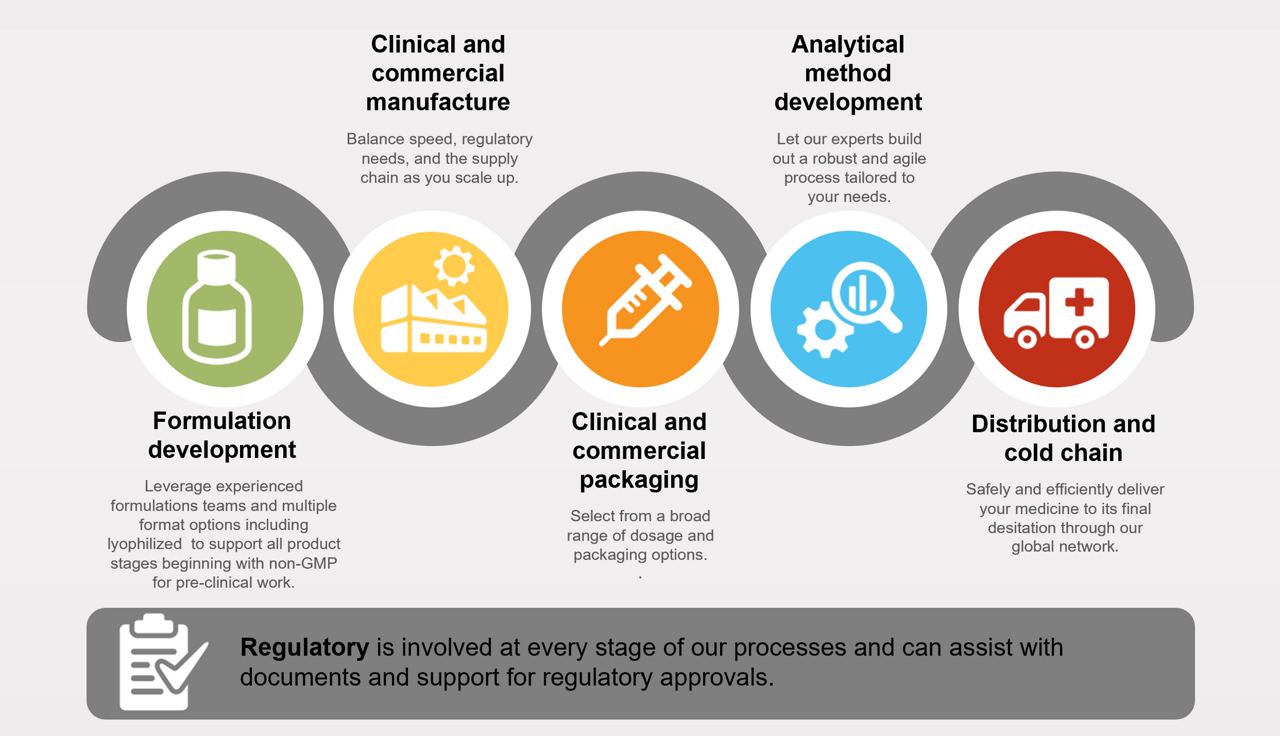
Improve your chances of approval. Patheon pharma services can help you balance speed, regulatory needs, and the supply chain as you scale up. We offer the ability to:
- Navigate a complex regulatory environment at a global scale
- Get your product to market and patients quicker
- Execute tech transfers with speed and efficiency
- Ensure a robust supply chain within a global network
Our experienced regulatory teams have an enviable track record of drug commercialization success for our clients. Leverage our commercial production and clinical trial solutions to gain extensive access to global technical experts, capability, regulatory insights, and transportation.
Learn more about our pharma commercialization services.


Whether you have a dosage format in mind or are exploring your options, we offer a variety of packaging choices. In one year, we produced more than 130 million sterile liquid and lyophilized vials and manufactured 40 dosage forms. As a result, we have many pre-qualified components and standardized processes.
We offer:
- Small- and large-volume parenterals
- Liquid-filled vials
- Lyophilized vials
- Extensive range of vial sizes including ISO standard
- Pre-filled syringes and cartridges
Pre-qualified components and standard processes
Thermo Fisher Scientific’s selection of product contact materials and components can shorten timelines, reduce capital expenditures, and eliminate cleaning validation, media fills, and container closure integrity (CCI).
Learn more about our clinical packaging and commercial packaging services.
Set your product up for long-term success throughout its lifecycle. Our experts can build out a robust and agile process tailored to your needs, including:
- Lyo-cycle process development and optimization
- Manufacturing process development studies:
- Mixing and pump-shearing
- Hold time studies
- Freeze-thaw studies
- Scalability studies
- Product contact part compatibility
- Proven Acceptable Ranges (PARs)
- International Council for Humanization (ICH) stability
- Shipping container selection
- Component compatibility testing
- Cleaning validation
- In-use stability
- Sterilization cycle development and validation

With more than 30 years of clinical trial experience, we can help your therapeutic arrive safely and on time. Whether your investigational medicinal product (IMP) is a traditional small molecule, biologic, or advanced therapy, we offer strategy, sourcing, management, packaging and labeling, storage, and distribution services. Visit our clinical trial services page for more information.
Cold chain services, logistics, and management
Know your products will reach their destination, intact and ready to use. Our global network provides comprehensive site-to-site connectivity with dependable storage, packaging, labeling, and shipment capabilities.
Learn more about our cold and ultra-cold supply chain management and logistics services.

Our sterile fill-finish global site locations
Our 642,000 sq. ft. campus in Monza, Italy is a center of excellence for sterile manufacturing. It features a unique co-location of mRNA manufacturing capabilities with LNP and fill-finish services to help streamline your processes and mitigate risks.
Our Greenville, NC facility is a large, multipurpose pharmaceutical manufacturing and packaging campus. This site provides both solid dose form manufacturing and packaging and sterile dose manufacturing, filling, and lyophilization of both biopharmaceuticals and small molecules.
Our Ferentino facility is 14,034 sq. m. (151,061 sq. ft.), specializing in integrated sterile liquid and lyophilized product development and commercial manufacturing, including high-potency products and LVP.
Our Swindon facility specializes in integrated sterile liquid and lyophilized product development and commercial manufacturing, including high-potency products and LVP. It offers extensive development and commercial capabilities for sterile dosage forms, including liquid vials, commercial fill-finish lines, and primary and secondary packaging vials.
Our state-of-the-art sterile drug development and manufacturing facility in Singapore supports rapid fill-finish vaccines and other therapeutics for an expanding biopharma market.
Helpful resources