Viral vector commercialization – Part 1:
Technology transfer processes
Category | Advanced therapy
The demand for viral vectors has significantly increased in recent years for their ability to efficiently deliver genetic material to target cells. This has led to the development of gene therapies and vaccines that have the potential to revolutionize medicine. However, viral vector manufacturing and commercialization require scientific expertise, technological know-how, and a solid infrastructure. This is where technology transfers play a critical role.
Viral vector tech transfer refers to the process of transferring knowledge, skills, and technology from one organization to another. They enable companies to develop and manufacture viral vectors at scale. They also help companies stay up-to-date on the latest advancements in the field and accelerate the development and commercialization of upcoming gene therapies and vaccines.
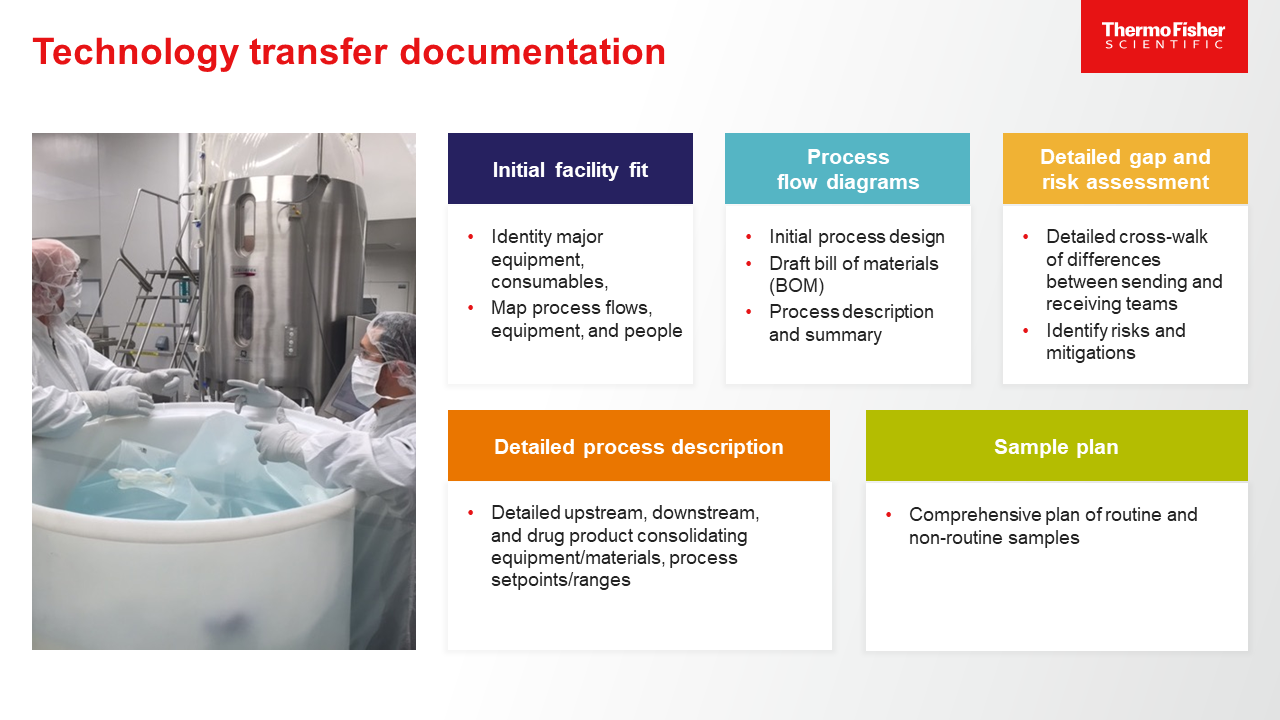
The key tech transfer documentation deliverables for viral vectors include:
- Initial facility fit
- Process flow diagrams
- Detailed gap and risk assessment
- Detailed process description
- Sample plan
We discuss the details of each deliverable and other technology transfer considerations in the video below.
To learn more about preparing viral vector productions for commercialization, check out our full webinar here.


