What’s the real challenge in biologic manufacturing scale-up? It’s not capacity—it’s demand
Key takeaways
- Demand uncertainty, not capacity, is the main barrier in biologics scale-up.
- Most programs never approach volumes that justify stainless-steel capacity.
- 5,000 L single-use systems offer flexibility when demand is unpredictable.
- A flexible, demand-led approach enables smarter investment and faster response.
Scaling a biologic isn’t difficult because capacity is scarce—it’s difficult because demand is unpredictable. By the time a program approaches late-stage development, teams must make high-stakes decisions about future volume needs long before the market provides clarity. Forecasts shift, clinical trial enrollment fluctuates, and competitive landscapes evolve. Yet drug developers are still expected to lock in manufacturing decisions that could shape the program’s trajectory for years.
Many biotech and biopharma companies assume the core risk in scale-up is finding enough capacity. But the real challenge sits earlier and runs deeper: predicting the demand curve well enough to avoid overbuilding or underbuilding your manufacturing strategy.
And this is exactly where a flexible platform, powered by 5,000 L single-use bioreactors (S.U.B.s), changes the story. It helps to reduce risk, remove constraints, and give sponsors the confidence to progress with a CDMO partner without waiting for perfect clarity.
Why capacity doesn’t have to be a constraint in biologics scale-up
Capacity may feel like a constraint, but in reality, most biologics never reach the volumes that require massive stainless-steel infrastructure. In fact, recent industry analyses show that the overwhelming majority of molecules in late-phase development fall within 2,000–5,000 L production needs (2023 CPHI Annual Report). Only a small subset ever justifies large, fixed assets.
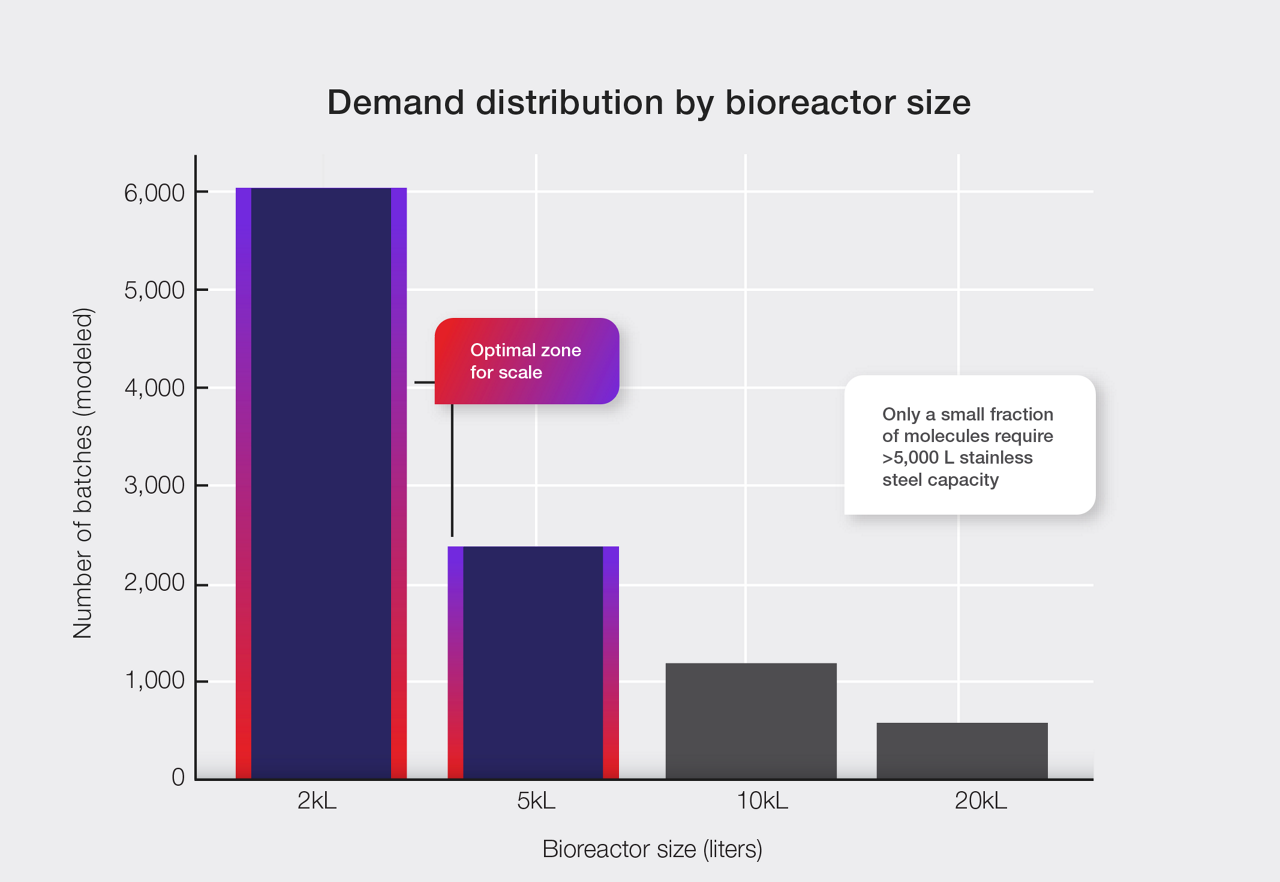
When demand data is plotted across today’s biologics pipeline, one pattern is clear:
Most late-phase molecules do not require large, fixed biomanufacturing installations.
This mismatch creates a persistent tension:
- Plan too big, and developers lock in capital and operational costs they may never need.
- Plan too small, and they risk falling behind the moment demand accelerates.
This middle zone—where most programs reside—is defined by uncertainty. And uncertainty makes every scaling decision a calculated risk. This means that most programs operate in a zone where flexibility offers more value than volume. Instead of planning for a high-volume future that may never arrive, developers can adopt a scalable model that adapts in real time to their sponsors demand needs.
What late-stage teams truly need isn’t more capacity. They need capacity that can flex with them.
With 5,000 L S.U.B.s, capacity stops being a bottleneck. Developers can scale up quickly when demand materializes—or scale down when forecasts shift—without committing to long validation timelines. This agility allows CDMOs to match capacity precisely to a sponsor’s evolving needs, not the other way around.
A CDMO’s role: Absorb uncertainty, not amplify it
For late-stage sponsors, the right manufacturing partner is one that can handle fluctuating needs without forcing premature decisions. A CDMO built on a 5,000 L single-use technology (S.U.T.) platform can:
- Add or remove capacity quickly
- Avoid the rigid timelines of stainless-steel builds
- Support seamless transitions between clinical and commercial batches
- Protect programs from delays when forecasts shift
Instead of anchoring production to fixed assets, the CDMO can match capacity precisely to sponsor needs—expanding when demand materializes and contracting when it doesn’t.
This dynamic model directly addresses the real risk developers face: not capacity scarcity, but capacity commitment.
How 5,000 L single-use systems reshape the scale-up equation
The 5,000 L S.U.T.—and specifically the HyPerforma™ DynaDrive™ single-use bioreactor—gives developers a way to scale without their sponsors having to assume long timelines, sunk costs, and operational inflexibility. It instead provides:
True flexibility: Scale up or down as demand evolves
The DynaDrive 5,000 L S.U.T. supports broad process transfer and predictable scale-up, enabling developers to:
- Move from early to late-stage production seamlessly
- Adjust capacity with less downtime and fewer constraints
- Respond quickly when clinical enrollment, market signals, or competitive dynamics shift
Two or more 5,000 L systems can also be multiplexed to reach high output levels—without sacrificing agility.
1 x 5,000 L S.U.B. for small to mid-volume programs or 2 x 5,000 L for large-volume programs
Cost-efficiency: Avoid overbuilding and reduce exposure
S.U.T. eliminates capital expenditures tied to steel infrastructure, as well as the cleaning, sterilization, and validation burden that slows programs down.
For developers, this means:
- Less upfront risk
- Faster deployment of capacity
- Lower operational costs
- The ability to “right-size” production continuously
This allows sponsors to invest when demand justifies it—not years before.
Sustainability: Modern expectations built in
Beyond operational advantages, the 5,000 L S.U.B.s reduces:
- Water use
- Energy consumption
- Facility footprint
- CIP/SIP utilities
It enables higher productivity per batch while decreasing overall resource intensity—aligning with the sustainability priorities many biotechs now factor into partner selection.
A More Adaptive Path to Biologics Growth
Uncertainty will always be part of biologics development. But with the right manufacturing strategy and CDMO partner, capacity and uncertainty in demand no longer needs to dictate the outcome.
A flexible, demand-driven model empowers sponsors to:
- Act with confidence even when forecasts evolve
- Reduce financial and operational exposure
- Keep programs moving through late-stage development without delay
- Capture opportunity when it appears—without overcommitting before it’s real
Thermo Fisher’s biologics CDMO services leverages S.U.B.s ranging from 50 to 5,000 L in scale, making this model possible. It transforms scale-up from a risky upfront commitment into a controlled, responsive, and strategically timed progression for every sponsor.


