The information you provide to the chat will be recorded to improve your experience and to contact you. Please read our privacy notice to see how we are processing and protecting your data. Click to view our Cookie Notice.
We'd love your feedback—take a quick survey to help us improve.
How can we help you today?
Search Patheon
Recent searches
Clear History
Suggestions
All Results
Thermo Fisher Scientific
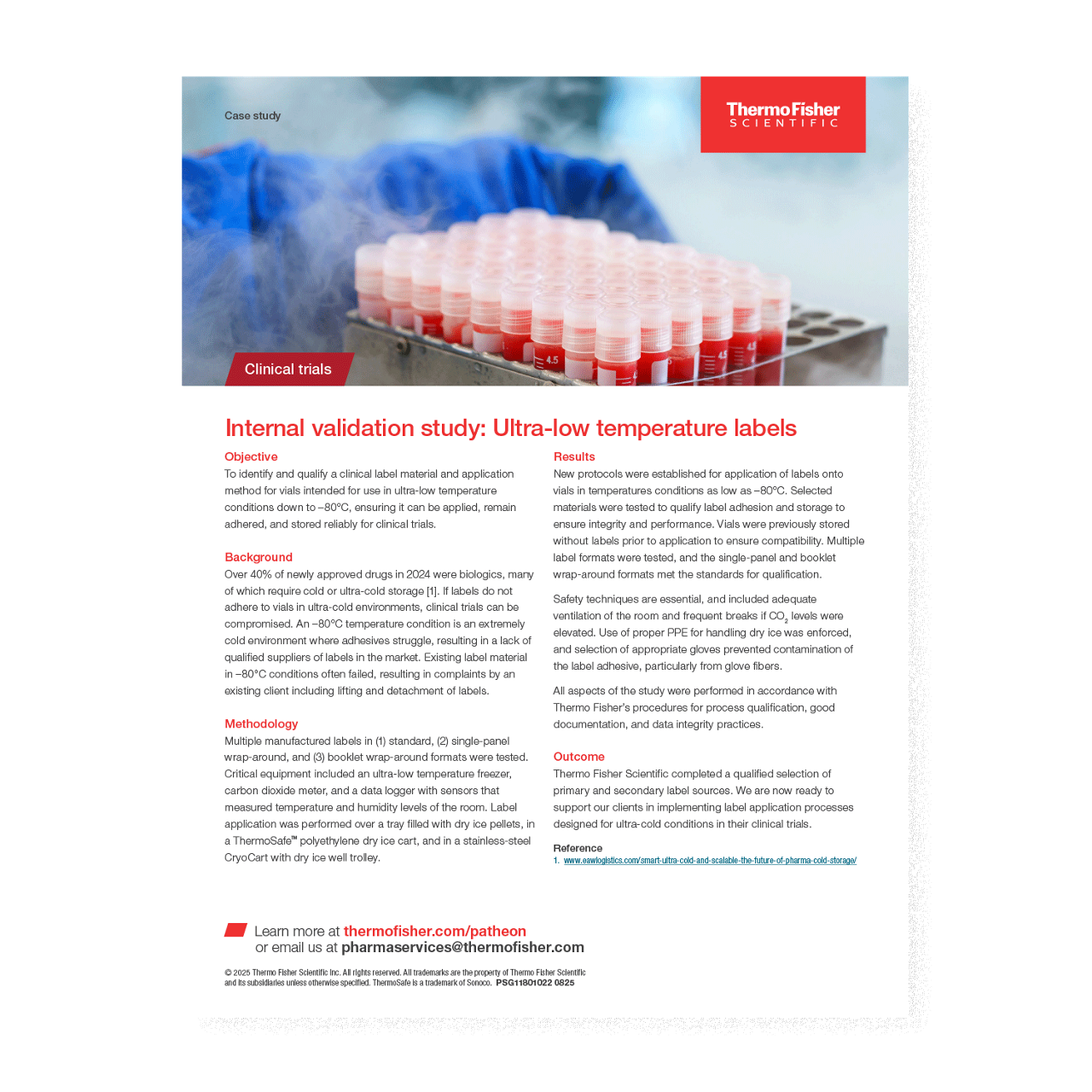
Over 40% of newly approved drugs in 2024 were biologics, many of which require cold or ultra-cold storage to temperatures as low as –80°C [1]. However, label adhesives often struggle in ultra-cold conditions, resulting in failures that can jeopardize clinical trials. With a lack of qualified suppliers of labels in the market, Thermo Fisher Scientific ran an internal validation study of its ultra-low temperature labels, in which multiple label formats were tested for their standards for qualification. Read the study to learn more.
[1] https://www.eawlogistics.com/smart-ultra-cold-and-scalable-the-future-of-pharma-cold-storage/