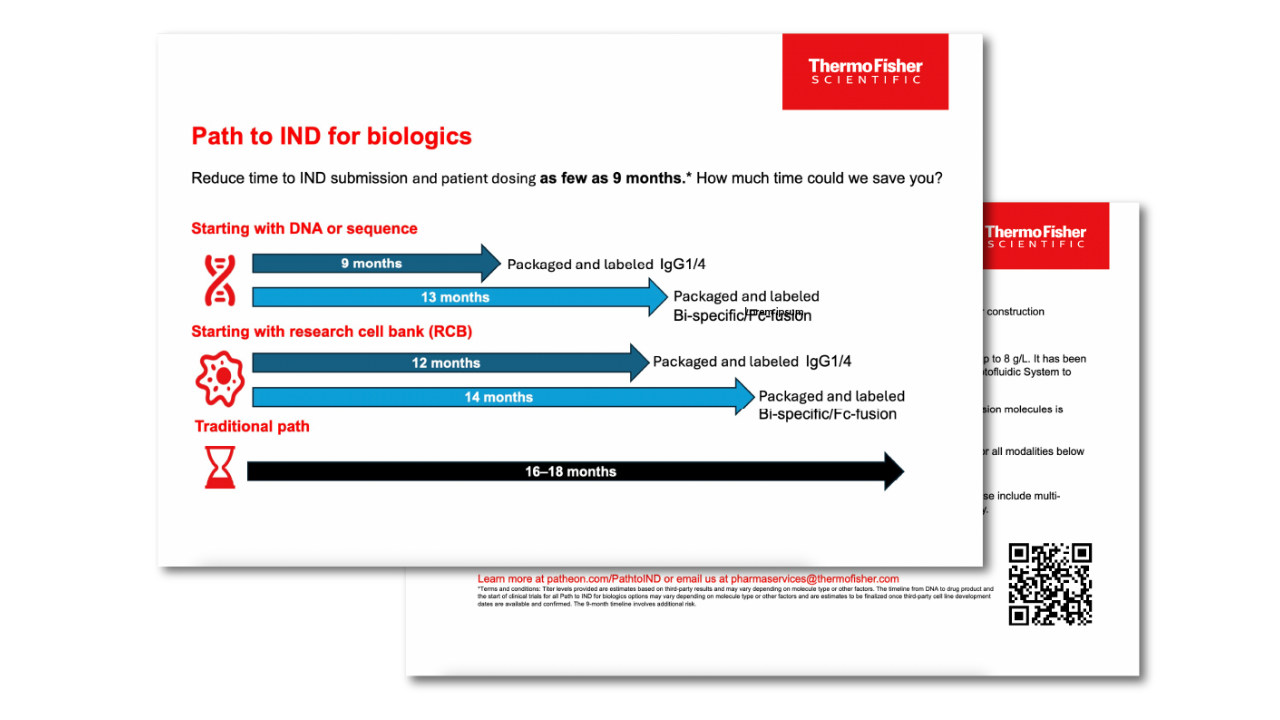
Path to IND for biologics: Go from DNA to drug product in as few as 9 months
Infographic
Path to IND for biologics delivers titer levels of up to 8 g/L* for mAbs, bispecifics, or Fc-fusion molecules. Powered by Thermo Fisher Scientific’s state-of-the-art equipment, cutting-edge software, and extensive resources—including raw materials and CRO services—this platform is designed to do more than develop your biologic. It accelerates your path to simultaneous IND/IMPD submission and Phase I clinical trial initiation, complete with funding and a ready-to-go drug product in as few as nine months.*
This infographic illustrates potential time savings based on project scope and starting materials. It also highlights the key steps that compress timelines while maintaining quality, including the use of our CHO K1 cell line with transposase technology, well-established platform formulations, comprehensive analytical testing services, and templated regulatory document packages. Together, we can take your clinically packaged biologic (IgG1/4) or bispecific/Fc-fusion molecule to market faster than you thought possible.