Clinical trial services
Global clinical supply solutions for every trial
At the center of every clinical trial is a patient waiting for a treatment to arrive safely and on time. In the highly competitive drug development market, biopharma companies face myriad challenges—from balancing cost, time, and quality to delivering the best possible outcome for their trial and their patients.
Our end-to-end global clinical supply chain services, formerly Fisher Clinical Services, are powered by people with an unwavering dedication to serving clinical research and patients around the world. Whether your investigational medicinal product (IMP) is a traditional small molecule, biologic, or advanced therapy, our more than 30 years of clinical trial services experience can provide support and expertise across your supply chain. We offer services for IMP, comparator, co-medication, and ancillary clinical supplies; including strategy, sourcing, management, packaging and labeling, storage, and distribution.
Download our interactive infographic to explore Patheon's end-to-end clinical trial service capabilities.
Our solutions offer the depth, breadth, and flexibility you need.
With more than 30 years of clinical trial experience and a breadth of services, Patheon can provide support and expertise across your supply chain.
Clinical trial services, solutions, and capabilities
With 35+ years’ experience in ultra cold chain management and logistics solutions we expertly safeguard the integrity of your advanced therapeutics and biological materials from storage to shipment, and everything in between.
Our comprehensive, end-to-end cold chain management solutions can support your advanced clinical trial at every stage, with services including:
- Biorepository
- Cold chain logistics and distribution
- Secondary packaging and labeling
- Kit design and production
- Laboratory processing
Helpful resources
We support clinical trials with an integrated supply chain and dependable global sourcing strategies. Complete with full packaging and distribution services, documentation support, and the highest product quality available, we strive to mitigate risk across the supply chain. Our Comparator Center of Excellence in Basel, Switzerland, includes dedicated teams in the United Kingdom, the United States, and strategic locations throughout Asia.
Comparator sourcing services highlights
- Sourcing strategy consulting and full-service procurement of drugs
- Regulatory expertise and market intelligence—trade and tax compliance with robust supplier qualification process and anticounterfeit procedures
- Sourcing for any comparator drug or IMP, reference medicinal products, matching placebo, rescue, background, co-medication, and standard of care
- Capability to source everything from one sample to large quantities for multi-year phase III trials either directly from the innovator, local, or open market sourcing
- Integrated services including clinical supply optimization, labeling, packaging, and distribution
Helpful resources
Our clinical manufacturing services for blinding, cGMP, and GAMP 5 (Good Automated Manufacturing Practice) for clinical trials provide a customized approach that includes a comprehensive offering aligned with regulatory and quality guidelines. We handle non-standard and challenging projects daily, so over the years, we have designed and manufactured specific tools and automation technologies to address unique processing needs.
Our standard blinding services through clinical manufacturing include:
- Blinding of pre-filled syringes, vials, bottles, nasal sprays, and IV bags
- De-blistering and de-bottling of globally sourced comparator drugs
- Fully automated blinding of comparators via single- and multi-product over-encapsulation
- High-speed manufacturing of matching placebos and inhalation capsules using Modu-C LS, Harro Höfliger
- Microdosing of APIs and API blends with Modu-C LS
- Special projects involving hazardous components
- Development of blinded comparators: method development and validation, stability testing, release, and IMPD submission data generation
Evolving clinical study designs compound complexity, cost, and timelines. Each trial has its own intricacies and special needs, often requiring ancillary supplies from multiple, unique suppliers. Let our team take your trial from startup to closeout in one seamless process. With our new clinical ancillary service model offering greater flexibility and cost efficiency, we can support clinical trials of every shape and size. Thermo Fisher Scientific can deliver when it matters most.
Our global clinical ancillary services include:
- Flexible sourcing and procurement
- Project management and trial oversight
- Supplemental clinical ancillary services
Helpful resources
Clinical trial label production requires a higher level of oversight and management than a commercial labeling operation to protect the integrity of blind, as well as meet stringent regulatory controls. Our advanced inspection software, full in-house print capabilities, translation and regulatory approval management process, and centralized web-based document management/routing platform, are specifically designed to reduce overall label cycle times.
Label services highlights
- Guidance on label setup, coordination of translations, regulatory review, and randomizations
- Full design capabilities (you only need to provide English text)
- Label design and manufacture integrated with regulatory, packaging, and clinical logistics
- Individual batch record control and release on a job-by-job basis
- Single- and two-panel labels, GlobalPly multilingual booklet labels, DigiPly digital booklet labels
- ATLASSM translations management system
A successful clinical trial is dependent on numerous factors, one of which is the effective planning and management of the clinical trial material supply chain. Our Enhanced Demand Planning service is a comprehensive service designed to manage, optimize, and streamline the clinical supply chain from early strategy development through the enrollment, maintenance, and closeout phases of a trial.
Our Enhanced Demand Planning services include:
- Early demand planning
- Forecasting and inventory management
- Simulation
- Inventory reporting and dashboards
- Proactive global project management
- Comprehensive development of supply strategy—minimizing risk and waste management strategies
- Hands-off supply chain management services
Helpful resources
Thermo Fisher’s fully owned cGMP facilities support ambient, refrigerated, and frozen packaging capabilities, and are strategically located around the globe to accommodate regional needs. An integrated IT system links the facilities to give clients control over inventory via bar code standards.
Our global clinical packaging services include:
- Primary packaging
- Secondary packaging
- ProSyriesSM pre-filled syringe assembly and labeling
- Smart packaging (medication adherence solutions)
Helpful resources
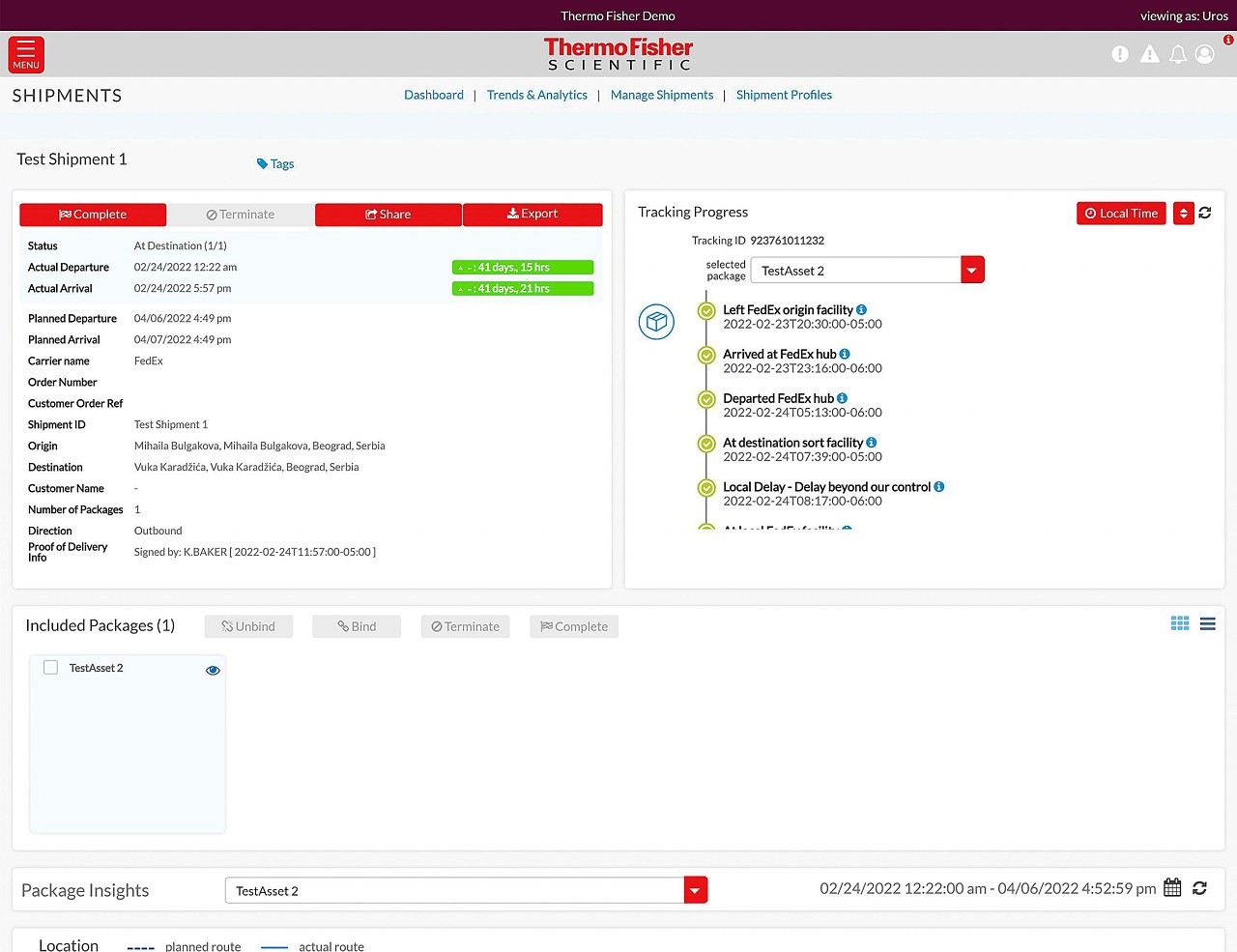
Enabling our customers to make the world healthier, cleaner and safer through enhanced shipment visibility
Global visibility anywhere, anytime
- Track all your shipments in one place
- Stay informed every step of the way
- View your cold chain shipments using real-time temperature and location tracking
- Track delays and excursions
- Advanced monitoring for critical cold chain and high value product shipments
Track and Trace digital portal
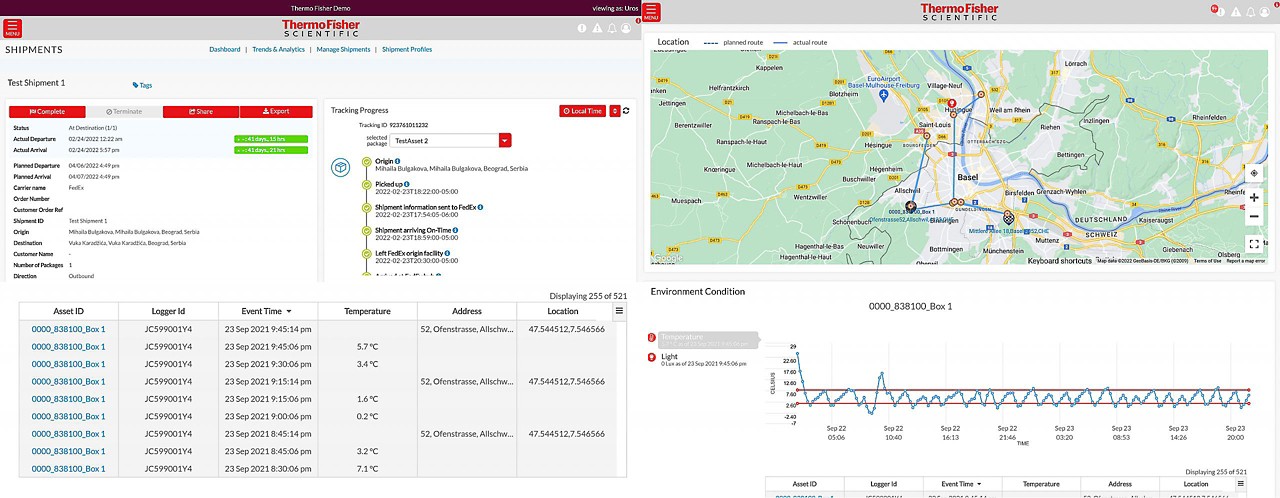
Track all your shipments in one place
Real-time sensors
View your cold chain shipments using real-time temperature and location tracking
For more than 30 years, we’ve supported the clinical trial supply, distribution, and logistics requirements of pharmaceutical and biotech sponsors worldwide. Our 27 purpose-built GMP/GDP compliant facilities, supported by more than 38 partner depots located across five continents, provide the global presence to support the regulatory-compliant movement, management, and delivery of supplies to more than 150 countries across all therapeutic indications. Our logistics experts handle the storage and distribution of labeled or packaged ambient and cold chain clinical trial materials, investigational medicinal products, comparator medicinal products and placebos, import/export services (including Importer of Record [IOR] capability in more than 24 countries to date), and returns and destruction of supplies across our network.
Storage, distribution, and clinical logistics highlights:
- Clinical trial material storage
- Total Transportation Management
- Real-time Track and Trace
- Cold chain logistics management
- Direct-to-Patient services
- Importer of Record (IOR) services (where available)
- European value-added tax (VAT) reclaim service
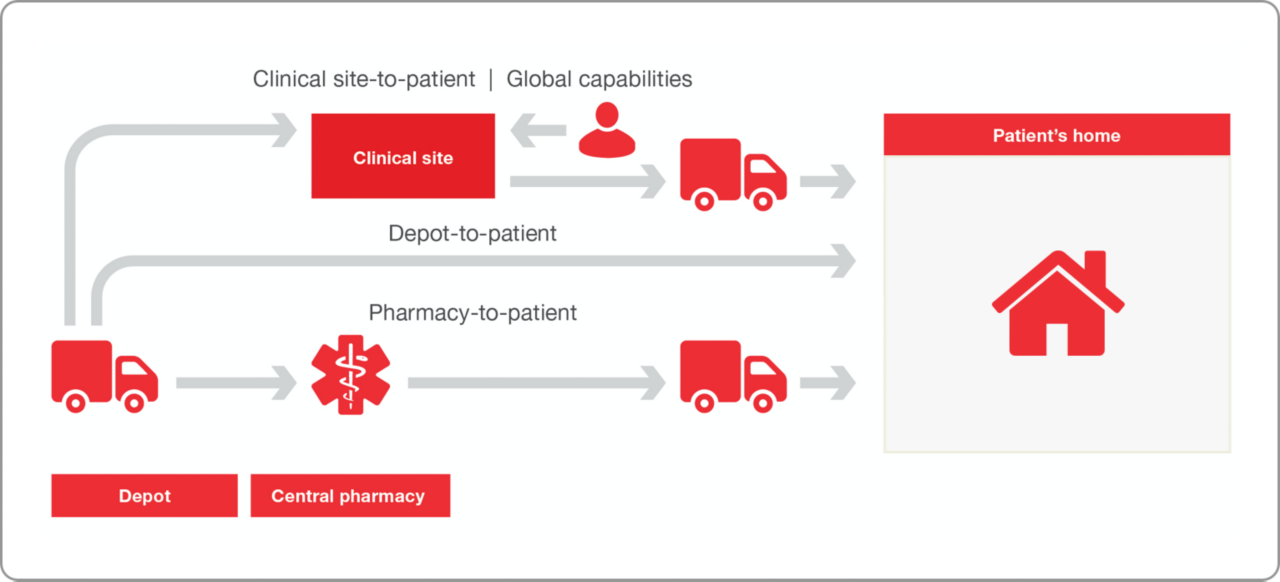
The typical clinical trial experiences a 30% patient dropout rate, which directly translates to increased risk and cost. According to patients, the study location and time spent on clinical visits are two of the top three dislikes of their overall clinical trial experience. These concerns can be eased or eliminated with decentralized clinical trials (DCTs).
DCTs have been an option for drug makers since the 1990s, and Patheon pharma services’ direct-to-patient offerings have been there from the start, with support now offered in more than 50 countries. Although DCTs are on the rise, there remain differing levels of comfort and readiness for pursuing DCTs, for not only drug developers but also the clinical sites and patients involved. That’s why we offer services to support traditional clinical trial channels, hybrid trials, and 100% decentralized trials.
- Compliant with applicable data privacy laws and regulations
- Global direct-to-patient service in more than 50 countries and the industry leading global network to support your studies in the rest of the world
- Nationwide shipments in the United States in all 50 states for IMP and non-IMP drugs
- Scheduled drug shipments and clinical ancillaries to the patient’s home
- Shipment coordination with patient, clinical site, and home healthcare nurse (if applicable)
- Solutions for IRT and non-IRT driven studies
- Established expertise with setting up decentralized clinical trials and patient compliance support
- Integrated CRO, IRT provider, clinical supply, and clinical operations teams that cuts your study start-up times to just a few months
Smart packaging (adherence measurement solutions)
In addition to DCT, we offer smart packaging capabilities to further help customers increase patient medication adherence. Smart drug packaging uses microcircuitry to passively measure dosing data and connect to the cloud using technology such as the patient’s smartphone. Cloud-based platforms then use sophisticated algorithms to analyze patient dosing behaviors and flag anything erratic so site staff can “rescue” patients and get them back to their regimen.
Smart packaging for medication adherence provides a rich and reliable data set to help understand patient dosing, making it applicable to most trial designs. It is especially useful for trials that:
- Require a precise understanding of time between doses
- Have complex dosing regimens
- Last longer than 1 year
- Include long inter-visit periods to clinical sites (decentralized clinical trials)
Helpful resources
The Global Gateway portal provides real time data transparency anytime and anywhere. It makes accessing inventory and distribution information of clinical trial supplies easy and fast for pharma and biotech companies.