Time to embrace electronic labels?
Potential labeling solutions under the new EU Clinical Trial Regulation
Category | Clinical trial services
The new EU Clinical Trial Regulation (CTR) is intended to simplify clinical trial administration and create a more welcoming climate for pharmaceutical companies that operate in Europe. As the regulation is now legally binding—unifying regulatory, labeling, and Qualified Person (QP) requirements—sponsors need to fully understand its requirements. Read on to learn implications for the drug industry, including routes to compliance, which requires experienced people, the ability to adapt, possibly a partner with expertise in clinical trials, a global footprint, proven technology, and risk mitigation skills.
Despite heated debate along the way, the CTR does not allow electronic labels (eLabels) for investigational drugs for clinical trials. Instead, printed expiry dates must appear on both the immediate and secondary packaging of investigational drugs, based on the traditional booklet label.
The CTR’s labeling requirements focus on the “period of use” indication—the expiry date of an Investigational Medicinal Product (IMP) or an Auxiliary Medicinal Product (AMP). This must appear on the product’s immediate or primary packaging and on its secondary packaging.1

In response to these new requirements, manufacturers have expressed concerns in multiple areas:
- Rework costs—These include costs to produce additional labels and associated project management and cabin fees. These are higher for kits with multiple primary containers.
- The appearance of tampering—This is a concern because compliance will often require breaking the tamper seals of the secondary packaging.
- Site capabilities—Since labeling activities must occur in a controlled cGMP environment, some sites may be unable to print, inspect, or even apply the labels.
- Cold product labeling—Medicinal products that must be maintained at deep cold temperatures present additional challenges for relabeling.
- Waste—Because of the difficulties involved in labeling primary packaging, there is an increased risk of damaged and discarded products.
Label provisions in EU Clinical Trial Regulation 536/2014:
What has changed?
The expiry date—or period of use indication—of IMPs or AMPs being supplied for clinical trials must now be shown on the immediate packaging, with no exceptions.2 This is required by Annex VI of the EU Clinical Trial Regulation 536/2014. This has a significant impact on the ability to perform shelf-life extensions and prevents possible use of electronic systems.
- Labeling separate supplies based on country and/or region
- Using a just-in-time approach for expiry
- Applying unique packaging designs, feeding the primary label out of the carton
- Using primary-only packaging
- Performing over-labeling and relabeling at the site and/or depot
- Pushing for better stability data/package at risk
- Implementing eLabel solutions
The last item on this list, eLabels, can take many forms, several of which can be updated rapidly via the cloud. Examples include the use of barcodes, quick response (QR) codes, radio frequency identification (RFID) tags, and electronic paper that is adhered to the packaging and can be changed (Figure 1).
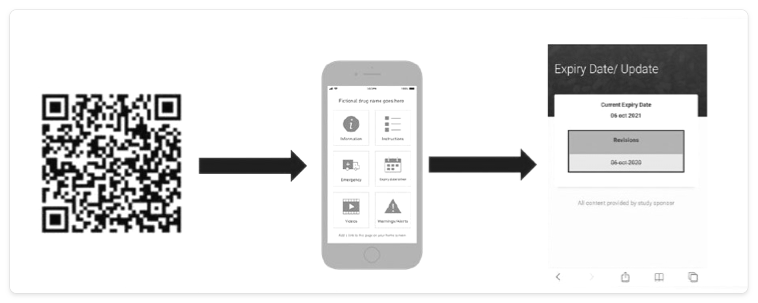
The basic functionality and benefits of eLabels are shown below.
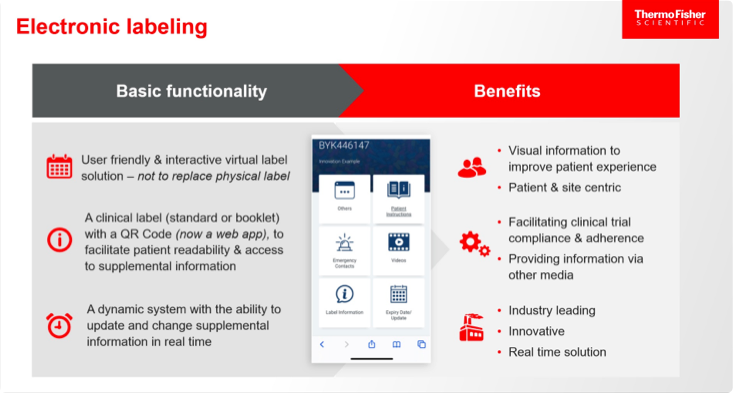
Benefits of eLabels for patients, sites, regulators, and sponsors, based on the findings of an initiative by TransCelerate—an organization that collaborates across the global biopharma R&D community on topics of common interest—also include the following:3
- Patients:
- Increases efficiency in clinical development, allowing patients to receive medicines more quickly
- Ensures greater patient safety
- Enhances utility of clinical labels and potential for better compliance, including availability of dosing videos, supplements to communication, and better usability
- Sites:
- Provides rapid access to real-time information
- Ensures greater efficiencies in labeling approaches
- Lays a foundation for future engagement with the patient about their medication
- Regulators:
- Decreases potential for deviations during extension re-stickering (e.g., relating to sterility, tamper evident seals, product mix-ups, or time out of environment)
- Makes latest information available for patients
- Ties into broader digital and innovation strategies
- Sponsors:
- Increases operational efficiencies in label creation
- Allows additional pooling strategies, which decreases waste
- Decreases reaction time to study changes
- Increases options for value-adds, including adherence programs, patient analytics, and patient education
Earlier efforts to expand eLabel use include a 2020 initiative by a group of seven major drug firms.4 This group, known as the Alliance to Modernize Prescribing Information, argued that paper labeling is outdated and incompatible with the technological flow of patient safety information. Others have pointed out that revisions to approved labels—which are needed an average of five times per year—are time-consuming and may involve a time lag, posing a patient safety risk.5 These arguments are valid, and it may at last be time to embrace eLabels for investigational drugs for clinical trials. One promising approach might be to start deploying electronic solutions on a small scale, in parallel with the required booklet labels, which includes patient information in the languages of all countries where the drug is available. If shown to offer continued, or even superior, protection for patient safety, this might provide the proof of concept needed to build trust among regulators.
However, patient safety must remain front and center at all times as new options are considered.
External partners can help fulfill sponsor needs for labeling compliance support, whether for individual elements to supplement a big pharma company’s own capacity or for a full range of end-to-end packaging and clinical trial services for an emerging biotech. Sponsors looking to partner on labeling advances should evaluate several qualities in potential partners:
- Exceptional people—These people should have deep labeling experience, ensuring successful delivery of labeling options to meet regulatory requirements for expiry dates.
- The ability to adapt—With a foundation in traditional approaches, an external partner should be able to pivot rapidly to adopt and integrate new approaches if required.
- Expertise in clinical trials—An experienced partner will bring close collaborative relationships with regulatory bodies to ensure sponsor compliance.
- A broad network—This should include a global footprint, bringing international contacts and expertise, and the ability to coordinate and centralize the many touchpoints involved in labeling.
- Proven technology—The external partner should bring approaches that have been vetted at global sites and depots, and validated for patient access.
- Risk mitigation and best-in-class quality control—This would address any concerns about access to information for patients, protecting electronic labeling elements from damage throughout the supply chain, and minimizing environmental waste.
Once the hurdle of the updated label requirements is overcome, sponsors can expect to gain the intended benefits of the new CTR, including improved collaboration, information sharing, and decision-making between and within member states.
View references
Category | Clinical trial services
The new EU Clinical Trial Regulation (CTR) is intended to simplify clinical trial administration and create a more welcoming climate for pharmaceutical companies that operate in Europe. As the regulation is now legally binding—unifying regulatory, labeling, and Qualified Person (QP) requirements—sponsors need to fully understand its requirements. Read on to learn implications for the drug industry, including routes to compliance, which requires experienced people, the ability to adapt, possibly a partner with expertise in clinical trials, a global footprint, proven technology, and risk mitigation skills.
Despite heated debate along the way, the CTR does not allow electronic labels (eLabels) for investigational drugs for clinical trials. Instead, printed expiry dates must appear on both the immediate and secondary packaging of investigational drugs, based on the traditional booklet label.
The CTR’s labeling requirements focus on the “period of use” indication—the expiry date of an Investigational Medicinal Product (IMP) or an Auxiliary Medicinal Product (AMP). This must appear on the product’s immediate or primary packaging and on its secondary packaging.1

In response to these new requirements, manufacturers have expressed concerns in multiple areas:
- Rework costs—These include costs to produce additional labels and associated project management and cabin fees. These are higher for kits with multiple primary containers.
- The appearance of tampering—This is a concern because compliance will often require breaking the tamper seals of the secondary packaging.
- Site capabilities—Since labeling activities must occur in a controlled cGMP environment, some sites may be unable to print, inspect, or even apply the labels.
- Cold product labeling—Medicinal products that must be maintained at deep cold temperatures present additional challenges for relabeling.
- Waste—Because of the difficulties involved in labeling primary packaging, there is an increased risk of damaged and discarded products.
Label provisions in EU Clinical Trial Regulation 536/2014:
What has changed?
The expiry date—or period of use indication—of IMPs or AMPs being supplied for clinical trials must now be shown on the immediate packaging, with no exceptions.2 This is required by Annex VI of the EU Clinical Trial Regulation 536/2014. This has a significant impact on the ability to perform shelf-life extensions and prevents possible use of electronic systems.
- Labeling separate supplies based on country and/or region
- Using a just-in-time approach for expiry
- Applying unique packaging designs, feeding the primary label out of the carton
- Using primary-only packaging
- Performing over-labeling and relabeling at the site and/or depot
- Pushing for better stability data/package at risk
- Implementing eLabel solutions
The last item on this list, eLabels, can take many forms, several of which can be updated rapidly via the cloud. Examples include the use of barcodes, quick response (QR) codes, radio frequency identification (RFID) tags, and electronic paper that is adhered to the packaging and can be changed (Figure 1).
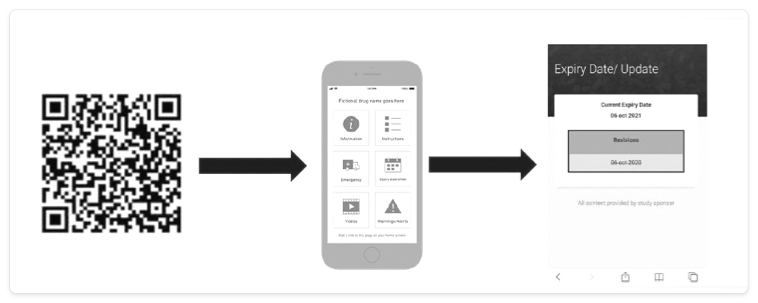
The basic functionality and benefits of eLabels are shown below.
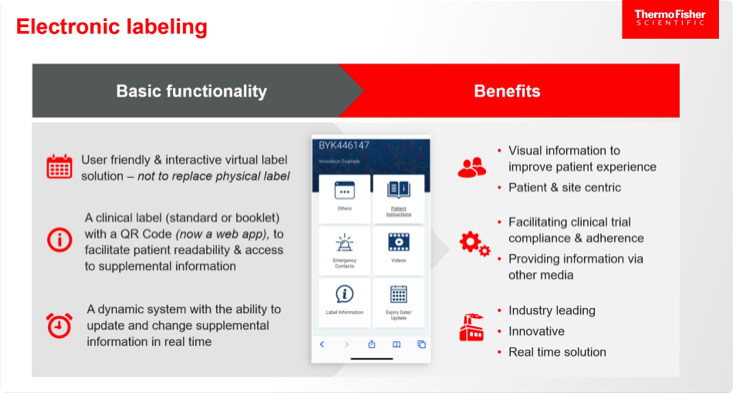
Benefits of eLabels for patients, sites, regulators, and sponsors, based on the findings of an initiative by TransCelerate—an organization that collaborates across the global biopharma R&D community on topics of common interest—also include the following:3
- Patients:
- Increases efficiency in clinical development, allowing patients to receive medicines more quickly
- Ensures greater patient safety
- Enhances utility of clinical labels and potential for better compliance, including availability of dosing videos, supplements to communication, and better usability
- Sites:
- Provides rapid access to real-time information
- Ensures greater efficiencies in labeling approaches
- Lays a foundation for future engagement with the patient about their medication
- Regulators:
- Decreases potential for deviations during extension re-stickering (e.g., relating to sterility, tamper evident seals, product mix-ups, or time out of environment)
- Makes latest information available for patients
- Ties into broader digital and innovation strategies
- Sponsors:
- Increases operational efficiencies in label creation
- Allows additional pooling strategies, which decreases waste
- Decreases reaction time to study changes
- Increases options for value-adds, including adherence programs, patient analytics, and patient education
Earlier efforts to expand eLabel use include a 2020 initiative by a group of seven major drug firms.4 This group, known as the Alliance to Modernize Prescribing Information, argued that paper labeling is outdated and incompatible with the technological flow of patient safety information. Others have pointed out that revisions to approved labels—which are needed an average of five times per year—are time-consuming and may involve a time lag, posing a patient safety risk.5 These arguments are valid, and it may at last be time to embrace eLabels for investigational drugs for clinical trials. One promising approach might be to start deploying electronic solutions on a small scale, in parallel with the required booklet labels, which includes patient information in the languages of all countries where the drug is available. If shown to offer continued, or even superior, protection for patient safety, this might provide the proof of concept needed to build trust among regulators.
However, patient safety must remain front and center at all times as new options are considered.
External partners can help fulfill sponsor needs for labeling compliance support, whether for individual elements to supplement a big pharma company’s own capacity or for a full range of end-to-end packaging and clinical trial services for an emerging biotech. Sponsors looking to partner on labeling advances should evaluate several qualities in potential partners:
- Exceptional people—These people should have deep labeling experience, ensuring successful delivery of labeling options to meet regulatory requirements for expiry dates.
- The ability to adapt—With a foundation in traditional approaches, an external partner should be able to pivot rapidly to adopt and integrate new approaches if required.
- Expertise in clinical trials—An experienced partner will bring close collaborative relationships with regulatory bodies to ensure sponsor compliance.
- A broad network—This should include a global footprint, bringing international contacts and expertise, and the ability to coordinate and centralize the many touchpoints involved in labeling.
- Proven technology—The external partner should bring approaches that have been vetted at global sites and depots, and validated for patient access.
- Risk mitigation and best-in-class quality control—This would address any concerns about access to information for patients, protecting electronic labeling elements from damage throughout the supply chain, and minimizing environmental waste.
Once the hurdle of the updated label requirements is overcome, sponsors can expect to gain the intended benefits of the new CTR, including improved collaboration, information sharing, and decision-making between and within member states.
View references


