Wherever you are, we are.
It was great to connect with you at CPHI Frankfurt 2025.
Thanks for visiting our booth to explore our 360° CDMO, CRO, and bioprocessing solutions. If you didn’t get the chance to talk with our team this year, it’s not too late. Contact us to learn how we can help streamline your drug development journey.
Amid economic pressures, regulatory shifts, and rising complexity, the need for reliable, agile partners had never been greater. At CPHI Frankfurt 2025, we brought together global expertise, integrated capabilities, and scientific depth to help our partners keep progress moving—wherever they were in the world, in their programs, or in the face of disruption.
Driving progress in drug development.
In our expert-led session, “What’s your next move—and what will it cost you?” Jennifer Cannon, president of commercial operations, shared how strategic integration across CDMO, CRO, and supply chain services can drive progress in drug development.
If you couldn’t attend—or want a quick recap of the key takeaways—check out our blog summary below.
Want to learn more about our industry-leading Accelerator™ Drug Development model? Check out our 360° CDMO, CRO, and bioprocessing solutions.
Let’s keep the conversation going.
CPHI is always a great way to kick off the holiday season and close out the year. But we’ve got big plans for 2026—and we hope you’ll join us.
We look forward to seeing you at other industry events in 2026. To view the full list of events we’ll be attending around the world, click below.
Find your missing element with Thermo Fisher Scientific
Insights and resources

Whitepaper
Transforming CDMO Partnerships Through Quality
Get an in-depth look into key indicators of CDMO quality, with tools and best practices to drive continuous improvement, strengthen collaboration, and ultimately cultivate trust.
View Infographic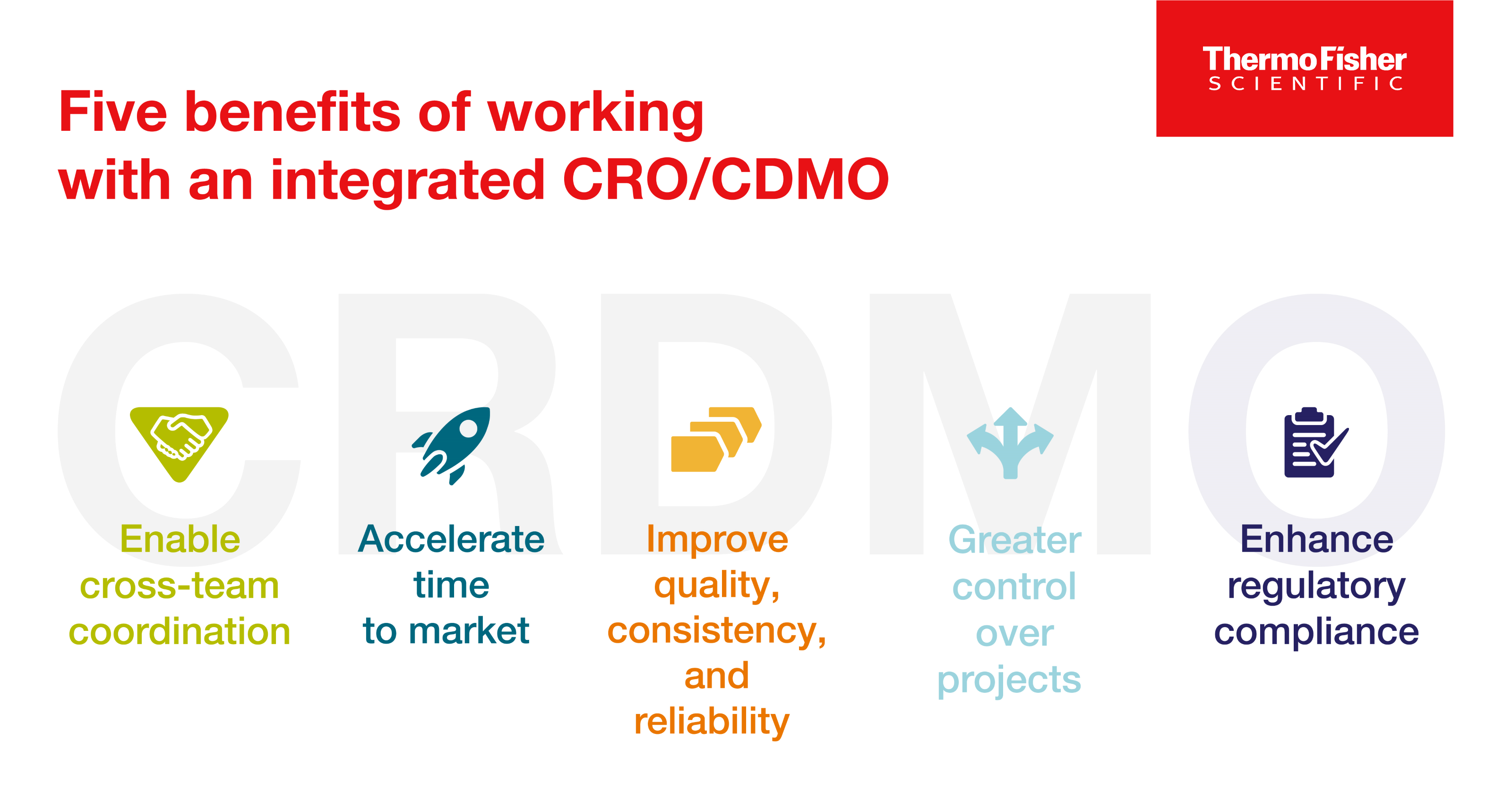
Blog
Enter the CRDMO: Reshaping drug development through CRO/CDMO integration
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog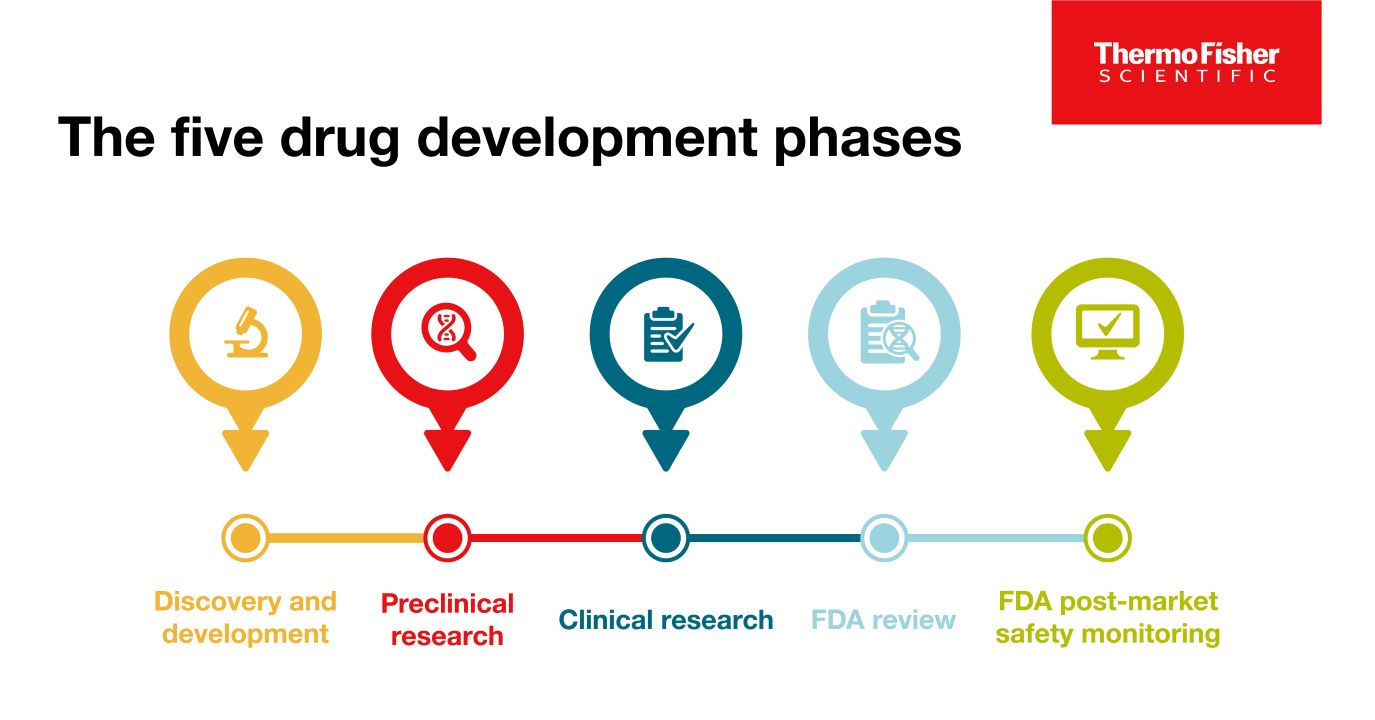
Blog
The 5 drug development phases
To be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) FDA review, and 5) safety monitoring.
Read Blog
Blog
CDMO 2.0: Three pharma industry trends for 2024 and beyond
Discover three major trends expected in the pharma industry, including turning to flexible manufacturing, embracing digital enablement, and the need for CDMOs deliver transformational value.
Read Blog
Blog
The quality lever: Shaping success in CDMO partnerships
Compromising on quality can lead to detrimental impacts on both speed and cost, ultimately affecting the successful development and marketing of new therapies.
Read Blog
Blog
Enter the CRDMO: Reshaping drug development through CRO/CDMO integration
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog
Blog
What is a CDMO? Seven things to look for in a quality CDMO partner
Learn how CDMOs (contract development and manufacturing organizations) work with pharma companies, and the top considerations companies have when choosing a CDMO partner.
Read Blog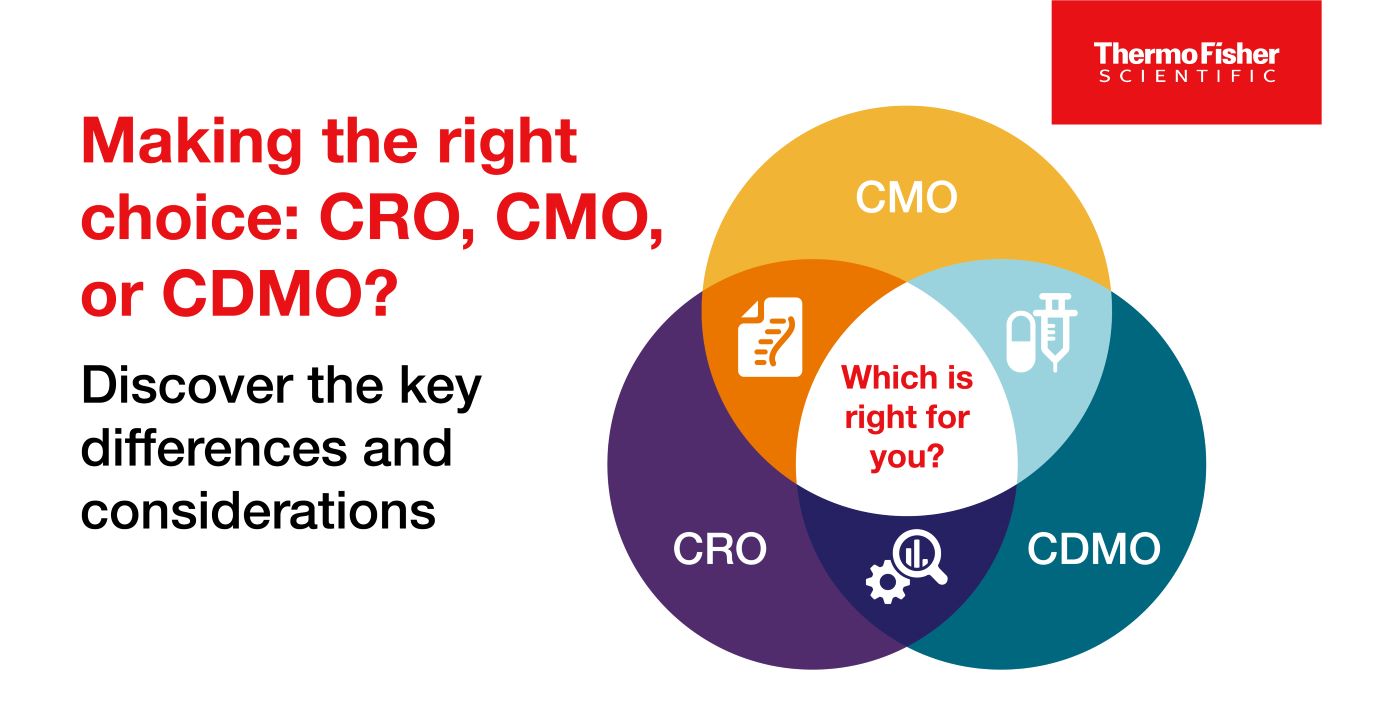
Blog
CROs vs CMOs, and CDMOs: What’s the difference between the three?
CROs, CMOs, and CDMOs all help biotechnology and pharmaceutical companies with drug development and manufacturing, but what’s the difference between the three?
Read Blog
Webinar
Optimizing the cell therapy patient journey through integrated CRO CDMO partnership
Watch this on-demand webinar for insights on how working with a single integrated CRO/CDMO partner can help ease industry challenges and provide an accelerated path from development to manufacturing, as well as the benefits that come from unified teams and infrastructure.

Infographic
CDMO Checklist to Launch Your Molecule Globally
Preparing to take your drug into the global market? You’ll need to make sure your CDMO has what it takes to successfully navigate the global regulatory space with speed, security, and supply safeguards. Use this quick list as a reference when evaluating your options.
View Infographic
Blog
Enabling a digital culture through integrated business processes
In contrast to a physical work environment, where stability and experience are key, a digital business environment focuses on innovation and connectivity. Learn more.
Whitepaper
The changing landscape of oncology drug development: Bringing novel lifesaving therapies to patients
Oncology is the fastest-growing, most active sector of drug development. Matching drug products to clinical and commercial needs requires scientific and technological innovation.
View Whitepaper
eBook
Protecting tomorrow: Supporting sustainability in the pharmaceutical and biotech industries
Learn how Thermo Fisher is meeting its environmental sustainability goals, and how we work in partnership with the pharma and biotech communities on shared environmental sustainability goals.
View eBook
Infographic
Preparing biologics for commercialization: Understanding Strategies to Reduce Risk and Optimize Outcomes in Drug Development
Within the drug development process, there are several steps that occur between the laboratory and final manufacture of the drug product. Different players step in during each point, so keeping a program with many moving parts on track requires planning and time-tested execution approaches.
View Infographic
Whitepaper
Advancing drug development using in silico modeling
This report provides a framework for that understanding by outlining some of the processes that stand to gain the most from computational modeling and identifying the in silico capabilities that can be used to accelerate and de-risk each phase of development.
Read WhitepaperDiscover a different kind of CDMO at CPHI
Stay connected to new discoveries, innovations, and expert scientific opinions.
Follow us on social media for the latest up-to-date event happenings.