CPHI Frankfurt 2025
The conversation starts here.
Amid economic pressures, regulatory shifts, and rising complexity, the need for reliable, agile partners has never been greater. At CPHI Frankfurt 2025, we’re bringing together global expertise, integrated capabilities, and scientific depth to help you keep progress moving—wherever you are in the world, in your program, or in the face of disruption.
Why visit Thermo Fisher Scientific at CPHI?
Whether you’re planning your first IND or managing commercial-scale production, Accelerator™ Drug Development, 360° CDMO and CRO solutions are designed to flex with your needs and reduce development friction. With a global network and a commitment to partnership, we help streamline transitions, shorten timelines, and provide clarity in a world of constant change.
Let’s talk about how we can help you:
- Navigate global complexity with geographic agility and regulatory foresight
- Build seamless pathways from early development through commercialization
- Adapt to evolving market demands without losing momentum
- Stay aligned across phases, functions, and teams
By connecting development, manufacturing, bioprocessing, and clinical supply services, we bring the clarity and continuity you need to move forward faster and with confidence.
Let’s start the conversation in Frankfurt.
Mark your calendar for this can’t-miss session

“What’s your next move—and what will it cost you? Driving value under pressure”
- Date: Wednesday, October 29, 2025
- Time: 1:30 – 1:55 p.m. CET (25 minutes)
- Location: Hall 4.1 - 4.1L8
Featuring Jennifer Cannon, President of Commercial Operations, Thermo Fisher Scientific.
In today’s high-pressure development landscape, every decision carries weight. From emerging biotechs navigating investment challenges on the path to IND, to established biopharma scaling complex programs toward commercialization, companies of all sizes are under increasing pressure to innovate, move fast, and deliver measurable value.
In this environment, traditional development models can slow progress, increase risk, and quietly drain time, money, and competitive advantage from a program. This session will examine a development model that’s reshaping expectations by unifying scientific, manufacturing, and clinical execution to unlock value at every stage.
Attendees will learn:
- Where disjointed execution models create risk, delays, and added cost in drug development
- What new data reveals about the ROI and timeline advantages of coordinated outsourcing
- How companies are adjusting execution strategies to maintain momentum under pressure
Be sure to attend our other panel discussions
Jennifer Cannon’s can’t-miss session is just the beginning. Thermo Fisher Scientific experts will also share their insights during two panel discussions on Tuesday, October 28, and Thursday, October 30.
Check out the full details below:
Panel #1
CDMOs at a crossroads: Scaling, adapting, and partnering for the future
Date: Tuesday, October 28, 2025
Time: 11:35 a.m. – 12:20 p.m. CET
Track: Hall 4.1-L8
Featuring Dr. Anil Kane, Ph.D., MBA, Global Head of Technical and Scientific Affairs, Thermo Fisher Scientific
The CDMO industry is at an inflection point. The explosive demand for GLP-1 therapies is straining manufacturing capacity, forcing rapid investment in specialized capabilities. Geopolitical tensions are disrupting supply chains, making regional diversification and de-risking strategies more critical than ever. Meanwhile, raw material shortages, rising costs, and labor constraints are challenging production efficiency—pushing CDMOs toward automation and digitalization. At the same time, pharma companies are no longer just looking for vendors; they need long-term strategic partners who can offer flexibility, scalability, and innovation.
Panel #2
Redrawing the pharma investment map: How trade, commercialization, and policy are reshaping capital flows
Date: Thursday, October 30, 2025
Time: 11:35 a.m. – 12:20 p.m. CET
Track: Hall 4.1
Featuring Kelly McGinnis, President of Business Operations, Thermo Fisher Scientific
Global pharmaceutical manufacturing is undergoing a fundamental shift, driven by rising trade frictions, evolving industrial policy, and strategic decoupling. Governments are increasingly focused on supply chain resilience, leading to targeted tariffs, reshoring incentives, and localized production mandates. At the same time, capital allocation is being reshaped by the growing influence of commercialization strategies—particularly around speed-to-market, IP protection, and regulatory harmonization across key regions.
Nowhere is this more evident than in the United States, where evolving drug pricing policy—especially around Medicare negotiations and international reference pricing—is creating new pressures on profitability and risk-adjusted returns. This policy uncertainty is prompting investors to reassess market-entry strategies and revisit the viability of previously dominant production geographies.
Together, these forces are redrawing the global pharma investment map—redirecting capital away from legacy hubs toward regionally aligned, politically secure ecosystems that offer both commercial advantage and policy predictability.
Find your missing element with Thermo Fisher Scientific
Insights and resources

Whitepaper
Transforming CDMO Partnerships Through Quality
Get an in-depth look into key indicators of CDMO quality, with tools and best practices to drive continuous improvement, strengthen collaboration, and ultimately cultivate trust.
View Infographic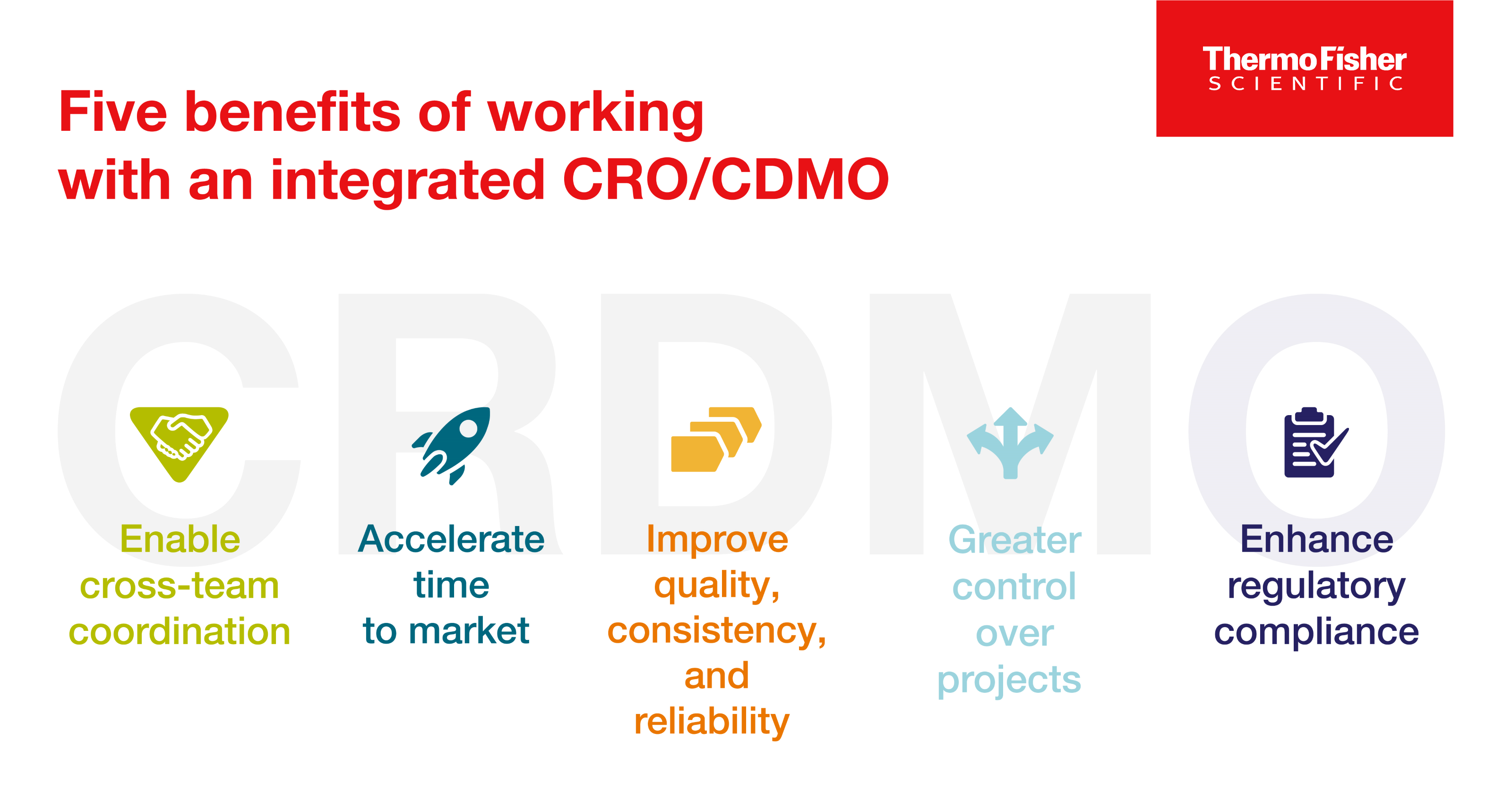
Blog
Enter the CRDMO: Reshaping drug development through CRO/CDMO integration
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog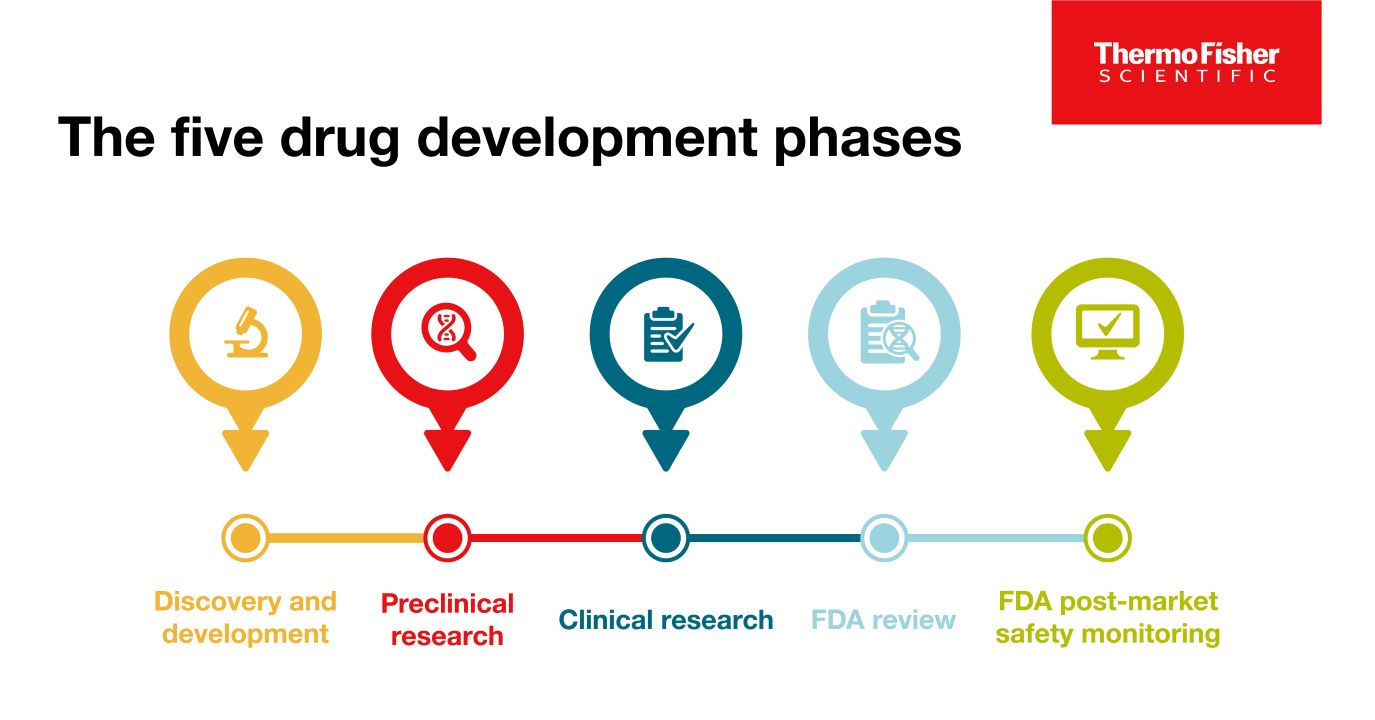
Blog
The 5 drug development phases
To be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) FDA review, and 5) safety monitoring.
Read Blog
Blog
CDMO 2.0: Three pharma industry trends for 2024 and beyond
Discover three major trends expected in the pharma industry, including turning to flexible manufacturing, embracing digital enablement, and the need for CDMOs deliver transformational value.
Read Blog
Blog
The quality lever: Shaping success in CDMO partnerships
Compromising on quality can lead to detrimental impacts on both speed and cost, ultimately affecting the successful development and marketing of new therapies.
Read Blog
Blog
Enter the CRDMO: Reshaping drug development through CRO/CDMO integration
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog
Blog
What is a CDMO? Seven things to look for in a quality CDMO partner
Learn how CDMOs (contract development and manufacturing organizations) work with pharma companies, and the top considerations companies have when choosing a CDMO partner.
Read Blog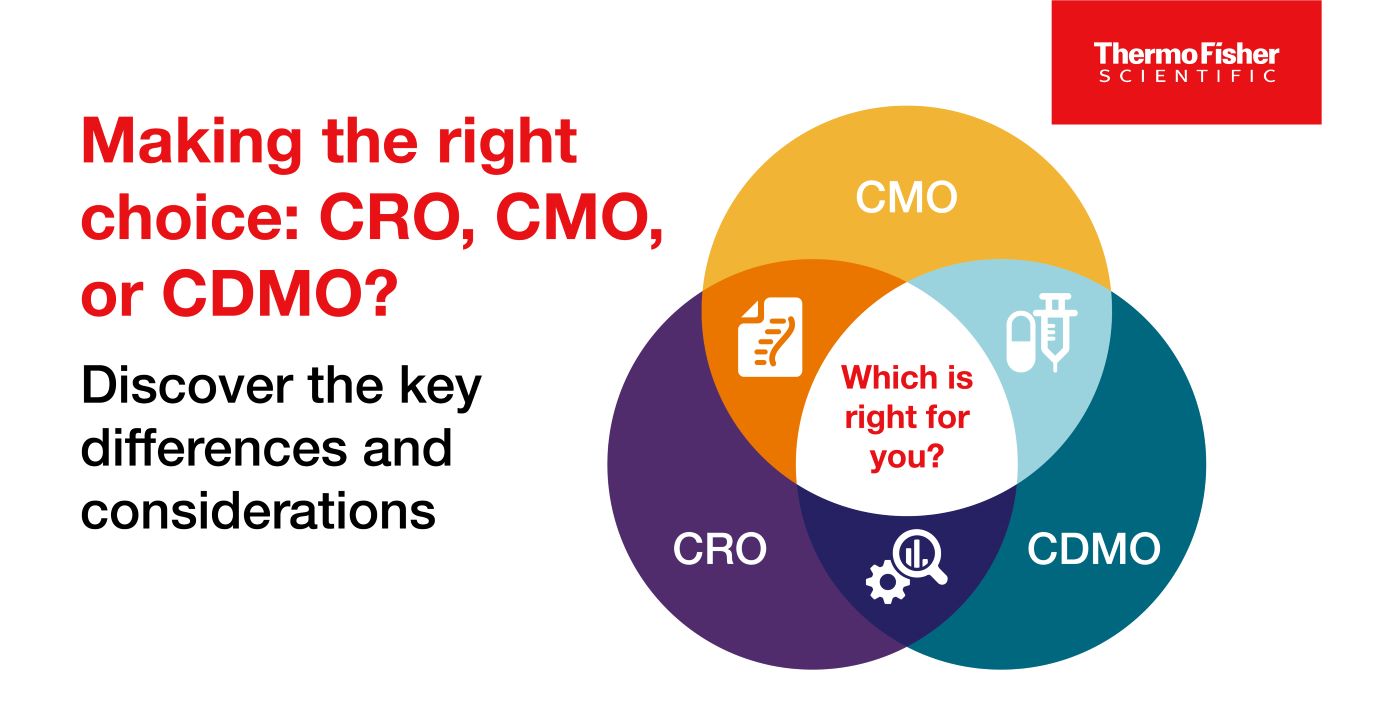
Blog
CROs vs CMOs, and CDMOs: What’s the difference between the three?
CROs, CMOs, and CDMOs all help biotechnology and pharmaceutical companies with drug development and manufacturing, but what’s the difference between the three?
Read Blog
Webinar
Optimizing the cell therapy patient journey through integrated CRO CDMO partnership
Watch this on-demand webinar for insights on how working with a single integrated CRO/CDMO partner can help ease industry challenges and provide an accelerated path from development to manufacturing, as well as the benefits that come from unified teams and infrastructure.

Infographic
CDMO Checklist to Launch Your Molecule Globally
Preparing to take your drug into the global market? You’ll need to make sure your CDMO has what it takes to successfully navigate the global regulatory space with speed, security, and supply safeguards. Use this quick list as a reference when evaluating your options.
View Infographic
Blog
Enabling a digital culture through integrated business processes
In contrast to a physical work environment, where stability and experience are key, a digital business environment focuses on innovation and connectivity. Learn more.
Whitepaper
The changing landscape of oncology drug development: Bringing novel lifesaving therapies to patients
Oncology is the fastest-growing, most active sector of drug development. Matching drug products to clinical and commercial needs requires scientific and technological innovation.
View Whitepaper
eBook
Protecting tomorrow: Supporting sustainability in the pharmaceutical and biotech industries
Learn how Thermo Fisher is meeting its environmental sustainability goals, and how we work in partnership with the pharma and biotech communities on shared environmental sustainability goals.
View eBook
Infographic
Preparing biologics for commercialization: Understanding Strategies to Reduce Risk and Optimize Outcomes in Drug Development
Within the drug development process, there are several steps that occur between the laboratory and final manufacture of the drug product. Different players step in during each point, so keeping a program with many moving parts on track requires planning and time-tested execution approaches.
View Infographic
Whitepaper
Advancing drug development using in silico modeling
This report provides a framework for that understanding by outlining some of the processes that stand to gain the most from computational modeling and identifying the in silico capabilities that can be used to accelerate and de-risk each phase of development.
Read WhitepaperDiscover a different kind of CDMO at CPHI
Continuing education for the pharma industry
Follow us on social for event updates