Thermo Fisher Scientific recognized by customers for contract manufacturing leadership
02/16/2024
Pharma and biotech customers distinguish excellence in capabilities, compatibility, expertise, quality and reliability
Category | CDMO Services
WALTHAM, Mass.--(BUSINESS WIRE)-- For the 11th consecutive year, Thermo Fisher Scientific’s pharma and biopharma customers recognized its contract manufacturing excellence through the annual CDMO Leadership awards sponsored by Outsourced Pharma and Life Science Connect. Thermo Fisher received five awards for capabilities, compatibility, expertise, quality, and reliability from three customer groups: big pharma, small pharma, and both.
In total, customers evaluated 98 companies across 23 performance metrics for these honors, which distinguish the top contract manufacturing organizations by surveying leading global pharma and biotech companies. That research, based on the Contract Manufacturing Quality Benchmarking conducted by Industry Standard Research (ISR), involved customers of all sizes that worked with the contract manufacturers during the prior 18 months.
“We’re honored by this demonstration of our customers’ trust and confidence in Thermo Fisher and very thankful for their recognition of our focus on exceeding expectations,” said Jennifer Cannon, president of commercial operations for the company’s pharma services business. “We’re committed to being the partner our customers rely on to help them deliver for patients around the world.”
Louis Garguilo, chief editor and conference chair, Outsourced Pharma, said, “Drug and therapy sponsors had much to be concerned with over the last 12 months or so. There was still supply-chain uncertainty, ever-growing novelty in platforms and processes requiring new skillsets and technologies, and the speed of everything seemed to accelerate. Through it all, CDMOs stayed focused, and also adapted to customer and market needs. Those best at serving their customers (and thus patients) are recognized as the 2024 CDMO Leadership Award winners. CDMOs may not have had all the answers in an uncertain year, but these are the external partners who provided the biopharma industry with the best solutions.”
Insights and Resources
Drug development and manufacturing is an ever-changing industry. Stay connected to new discoveries, innovations, and expert scientific opinions.

Blog
What is a CDMO? Seven things to look for in a quality CDMO partner
Learn how CDMOs (contract development and manufacturing organizations) work with pharma companies, and the top considerations companies have when choosing a CDMO partner.
Read Blog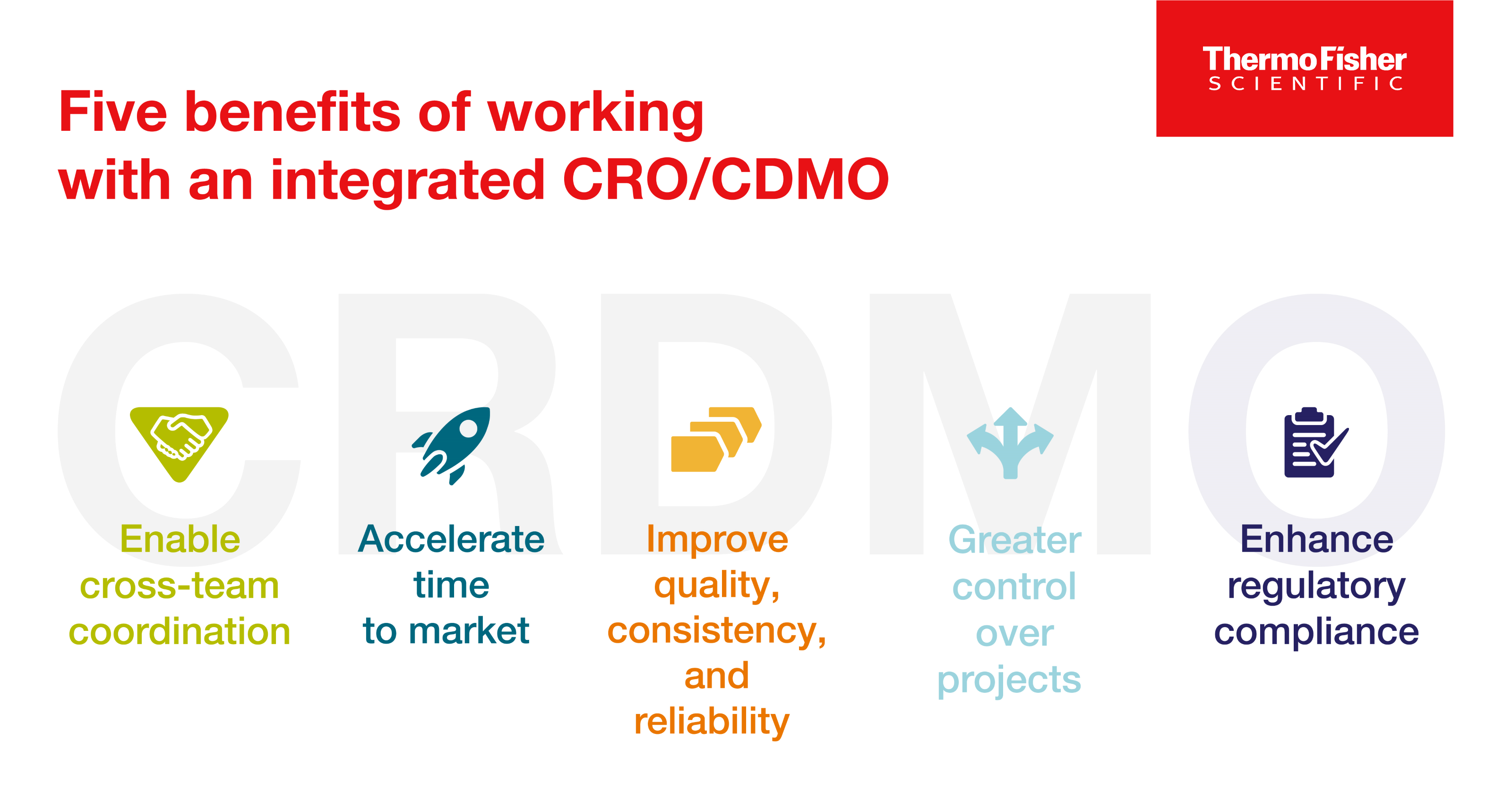
Blog
Exploring CRDMOs: Reshaping research, development, and manufacturing
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog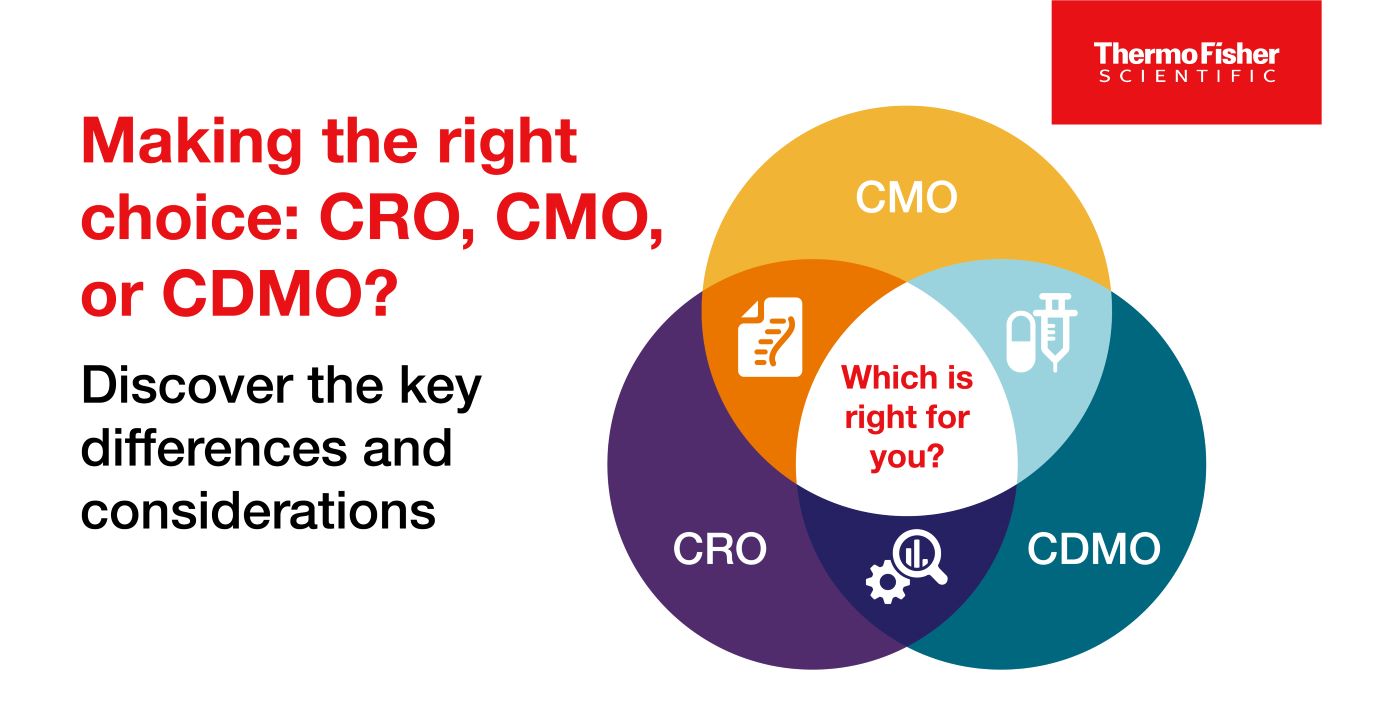
Blog
CROs vs CMOs, and CDMOs: What’s the difference between the three?
CROs, CMOs, and CDMOs all help biotechnology and pharmaceutical companies with drug development and manufacturing, but what’s the difference between the three?
Read Blog
Blog
The quality lever: Shaping success in CDMO partnerships
Compromising on quality can lead to detrimental impacts on both speed and cost, ultimately affecting the successful development and marketing of new therapies.
Blog
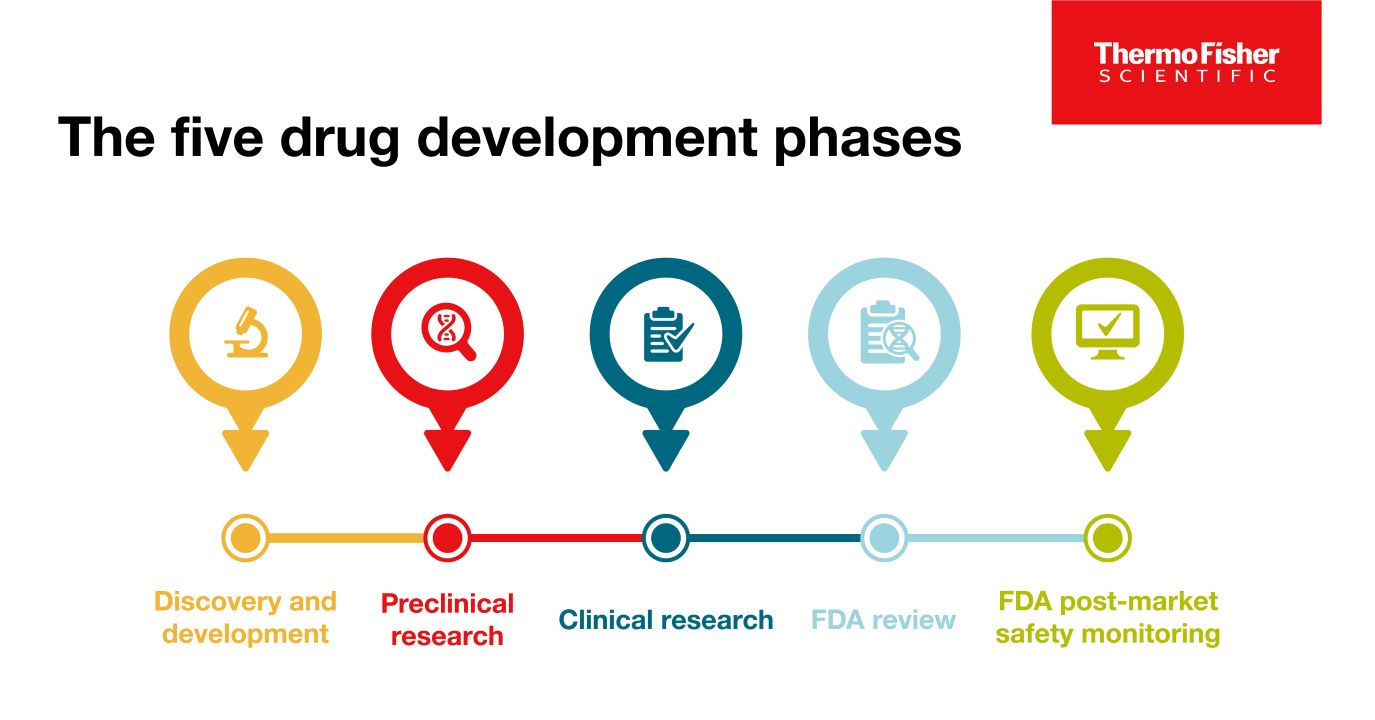
The 5 Drug Development Phases
To be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) FDA review, and 5) safety monitoring.
Read Blog
Blog
Exploring four patient-centric trends shaping today’s biopharma landscape
The biopharma industry is adopting a patient-centric approach to drug research, development, and manufacturing. Explore four trends shaping today’s landscape.
Read Blog
Blog
CDMO 2.0: Three pharma industry trends for 2024 and beyond
Discover three major trends expected in the pharma industry, including turning to flexible manufacturing, embracing digital enablement, and the need for CDMOs deliver transformational value.


