Advancing clinical trials with proven supply chain solutions
Helping drug developers reduce risk and maintain continuity through efficient,
resilient supply chain services
Keeping clinical trials moving forward
Drug developers worldwide are under pressure to keep their clinical trials on track—navigating global regulations, proactively managing risks, and safeguarding product integrity, all while meeting critical milestones. The right supply chain partner is essential to avoiding costly delays that may compromise the success of your research study.
Thermo Fisher Scientific provides flexible, end-to-end clinical trial supply chain services designed to address these challenges head-on. Our global network offers full-program support—from cold chain management to ancillary material sourcing, logistics, and secure warehousing—so you can advance through each phase with confidence.
One partner for sponsors of all sizes
Support across the Americas (54%), EMEA (26%), and Asia-Pacific (19%).
Helping drug developers navigate regulatory requirements across regions to safely bring their products to patients worldwide.
Assisted with 620 dossiers (writing and review) for 241 products (2020–2024).
Extensive experience preparing and reviewing clear and compelling dossiers to support hundreds of product approvals.
A leading CDMO: 34 non-NME NDA approvals secured between
2015 and 2024.
We receive more contracts for non-NME DNA approvals than any other CDMO—you can trust us to take care of your program.
Today’s pharma supply chain challenges
Drug developers face growing complexity across the global supply chain, including:
- Intense regulatory scrutiny and compliance demands across regions
- Rising costs and constrained capacity for critical clinical materials
- Vulnerability to geopolitical events, policy shifts, and natural disasters
- Growing risks in cybersecurity, data integrity, and supplier reliability
- Pressure to adopt technologies such as AI/ML and real-time tracking
To help navigate these challenges, Thermo Fisher Scientific applies a proven 3-step approach to supply chain risk management: Identify, quantify, mitigate.
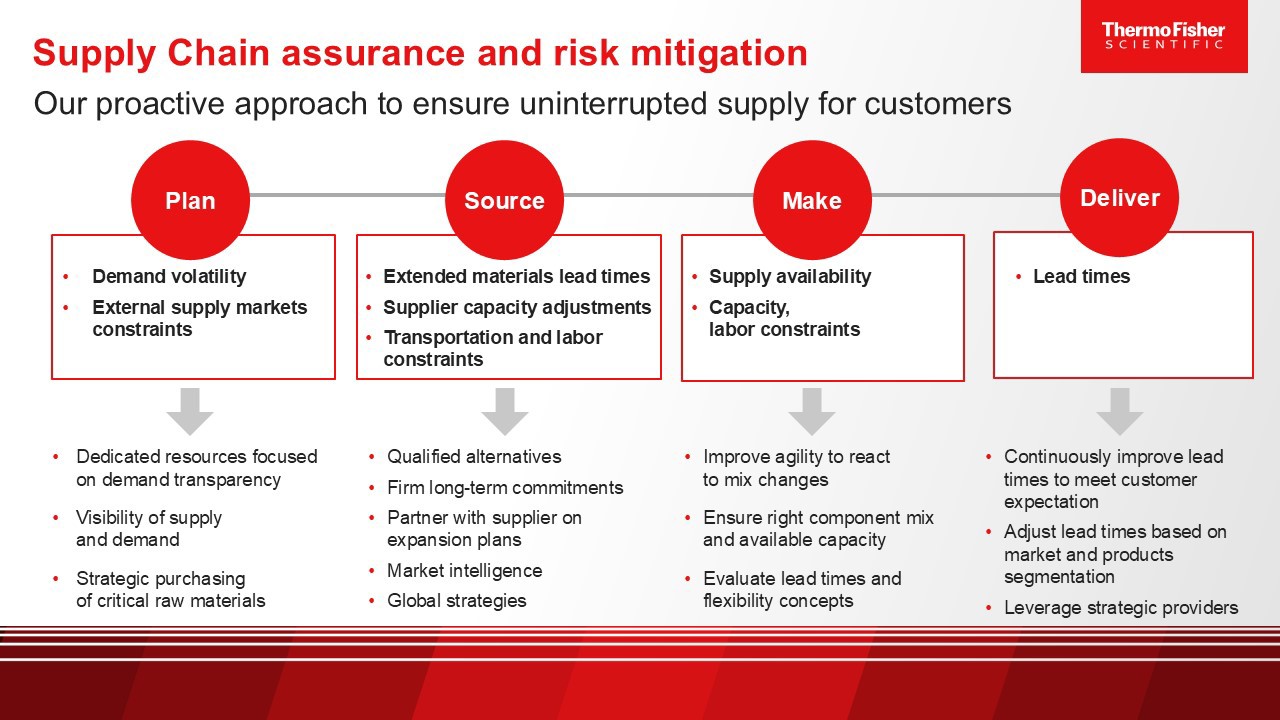
Supply chain risk management (SCRM)
Reduce supply chain risk with our proven 3-step process: Identify, quantify, mitigate.
Proactive
- Flag sole or single-source suppliers, long lead times, and regulated items
- Evaluate financial, EH&S, cybersecurity, and socioeconomic vulnerabilities
- Assess natural disasters or geopolitical events that could disrupt supply
Reactive
- Continuously track and monitor the supplier network for events that could impact operations
Proactive
- Prioritize risks based on their likelihood and potential for impact
- Determine which risks can be reduced through early intervention
- Model different risk scenarios to guide strategic decision-making
Reactive
- Follow standard playbook with clear roles, responsibilities, and escalation/de-escalation procedures
Proactive
- Develop and document successfully implemented strategies
- Apply mitigation at the local, divisional, and enterprise levels
Reactive
- Conduct post-incident reviews and refine processes accordingly to prevent future recurrences
Efficient, resilient strategies for stronger trials
We are committed to transparent communication, continuous improvement, and proactive updates at every stage of your clinical trial. During the COVID-19 pandemic, our network experienced no major disruptions—demonstrating our ability to safeguard supply chain integrity under pressure.
Today, we continue to invest in capabilities that strengthen your clinical trials, including:
- Direct-to-patient and site-to-patient delivery models
- Digital enablement for audits, monitoring, and visibility
- Data analytics, artificial intelligence (AI), and automation
- Industry-leading cold chain infrastructure for biologics
Supporting every component of clinical supply
Access our world-class network of facilities
Solid dose manufacturing and packaging, plus sterile dose manufacturing, filling, and lyophilization for both biopharmaceuticals and small molecules.
Explore our facility in Greenville, North Carolina, USA.
Comprehensive suite of sterile fill-finish and viral vector services, spanning from process development to scale-up for clinical and commercial manufacturing.
Explore our facility in Plainville, Massachusetts, USA.
Flexible drug substance manufacturing for highly potent compounds, including process development, scale-up, and large- and mid-scale API production.
Explore our facility in Florence, South Carolina (East), USA.
Process development and chemical production of intermediates and APIs for toxicology, preclinical, and clinical supply, with a potency capacity of Cat 3a.
Explore our facility in Florence, South Carolina (West), USA.
Center of excellence for innovative bioprocessing and single-use technology (S.U.T.), including our HyPerforma™ DynaDrive™ Single-Use Bioreactors (S.U.B.s).
Explore our facility in St. Louis, Missouri, USA.
OSD center of excellence focused on solubility enhancement, advanced drug delivery, and early-stage development, supporting preclinical to Phase IIb.
Explore our facility in Bend, Oregon, USA.
Offers a range of OSD development and manufacturing services, allowing biotech and pharma companies to stay in one place as their programs progress.
Explore our facility in Cincinnati, Ohio, USA.
A softgel center of excellence since 1996, providing extensive development and commercial-scale capabilities across a variety of softgel technologies.
Explore our facility in High Point, North Carolina, USA.
Medium- to high-volume commercial manufacturing of solid dosage forms, including tablets, capsules, bulk granules, and powders packaged in bottles.
Explore our facility in Manatí, Puerto Rico, USA.
State-of-the-art sterile fill-finish and packaging site for drug developers, with expanded capabilities in pre-filled syringe and liquid vial manufacturing.
Explore our facility in Ridgefield, New Jersey, USA.
Learn more through our helpful resources
Building resilience and continuity
Global reach and local expertise
Ready to strengthen your clinical supply chain?
We’ve worked with hundreds of sponsors at all stages of development to strengthen their clinical supply chains. Together, let’s navigate challenges and overcome complexity—ultimately getting treatments to patients faster.

