In-silico modeling services
Enabling smarter decision making, accelerated R&D, and risk mitigation
In silico modeling technologies are transforming drug development and manufacturing by enhancing efficiency, accuracy, to allow innovations. Powered by artificial intelligence, machine learning, bioinformatics, molecular dynamics simulations, and systems biology, these tools help accelerate the development of new drugs, optimize their formulation, ensure quality in manufacturing, and streamline regulatory processes, all while potentially reducing costs and time to market.
OSDPredictTM: Predictive modeling for smarter, API-sparing drug formulation
OSDPredict is a digital toolbox powered by AI/ML that helps biotech innovators solve formulation challenges before they become costly setbacks.
By combining multiple predictive models, including Thermo Fisher Scientific’s Quadrant 2™ platform, OSDPredict delivers data-driven foresight into solubility, permeability, bioavailability, FIH dosing, packaging, and scale-up planning.
Instead of trial-and-error, researchers can focus solely on what works, saving valuable API, compressing timelines, and reducing risks.
- AI and machine learning-enabled predictive modeling for solubility and bioavailability enhancement
- Accelerated stability modeling for shelf life and packaging determination
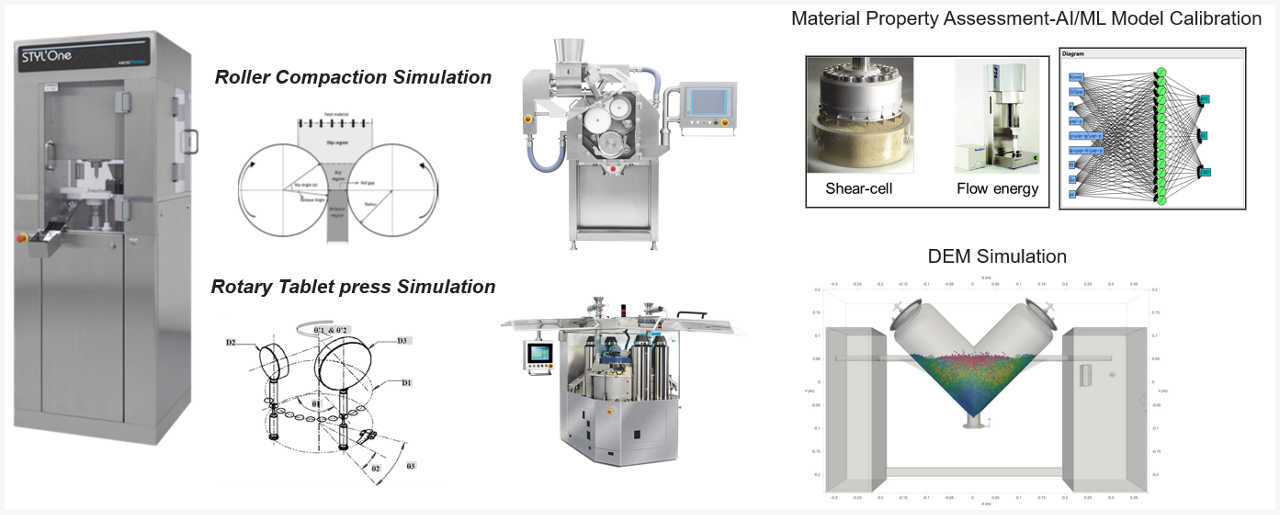
- Materials science, compaction simulation, and process modeling
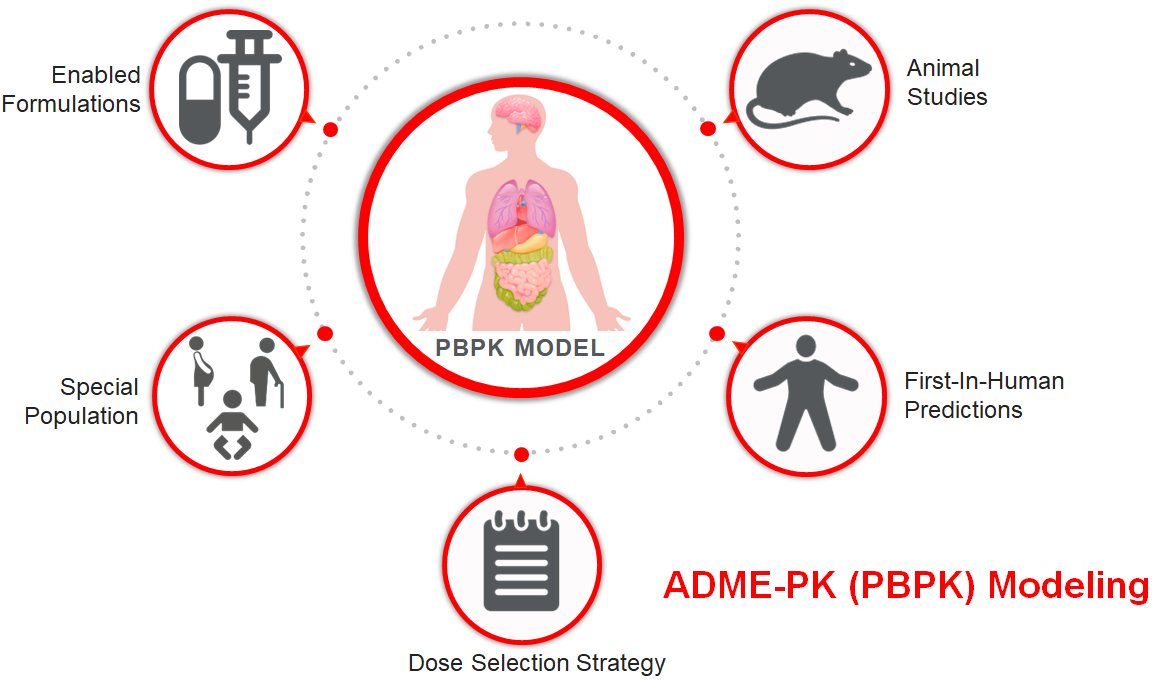
- ADME-PK modeling to predict the effect of API physiochemical traits on its pharmacokinetics
Key applications of OSDPredict
- Formulation behavior prediction—anticipate solubility, permeability, and bioavailability issues before they derail programs
- First-in-human dose optimization—model FIH dosing with confidence and clarity
- Packaging selection—identify the right packaging strategy to ensure stability and compliance
- Regulatory data generation—support IND-enabling studies with predictive insight
- Scale-up planning—plan manufacturing transitions earlier with higher probability of success
What are the benefits of using OSDPredict?
- Save precious API—minimize experimental waste and conserve scarce molecules.
- Rapid advancement of early development—reach milestones faster with predictive foresight
- De-risk decision making—act with confidence, guided by AI-driven predictions
- Increase first-time success—reduce reformulation cycles and achieve IND readiness smoothly
Digital modeling powered by AI, machine learning, and other innovative technologies, are transforming every stage of drug development by enhancing efficiency and precision, minimizing risks, and accelerating progress.
In silico modeling services, solutions, and capabilities
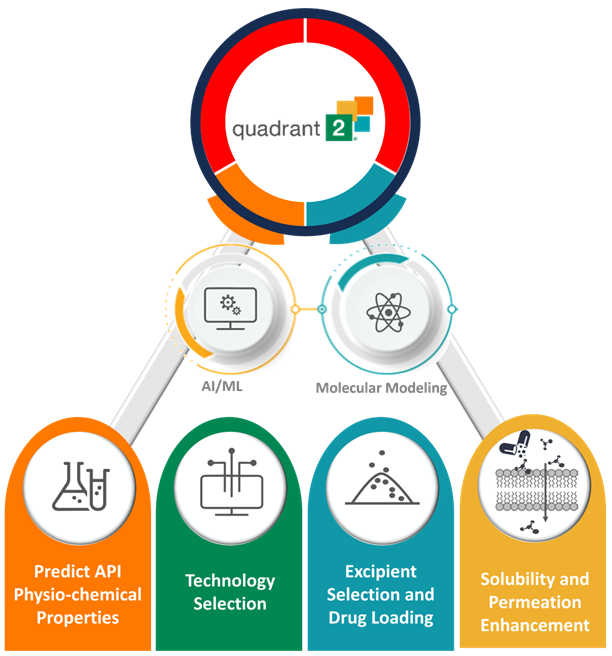
Between 70% and 90% of new chemical entities in development face solubility challenges, affecting bioavailability. Addressing these issues requires knowledge of delivery mechanisms and excipient functionality. Early consideration of formulation strategies affecting bioavailability and solubility is important to avoid costly errors later. Thermo Fisher Scientific’s proprietary platform for solubility and bioavailability enhancement, Quadrant 2, is a diagnostic tool for early development, which allows us to see in-silico predictions of formulations, which can save time and costs compared with trial-and-error approaches
Quadrant 2
Learn about our proprietary platform for solubility and bioavailability enhancement, Quadrant 2TM here.
Helpful resources
Browse our resource library to learn more about Quadrant 2 and other digital modeling capabilties.
Determining product shelf life is a regulatory requirement for pharmaceuticals and an important consideration for packaging decisions.
Predictive accelerated stability studies allow the long-term stability characteristics of a drug substance or drug product to be characterized from extrapolation of results of short-term studies that measure, track, and quantify stability-indicating attributes, such as degradation, thermal properties, crystallinity, color, viscosity, and particle size.
Computational methods for accelerated stability assessment program (ASAP) studies are powerful tools for quickly and accurately predicting product shelf life and packaging options for tablets, capsules, softgels, intermediates, granules, blends, solutions, and suspensions.
Predictive stability modeling is widely accepted globally for early clinical trials (INDs/IMPDs. The data are also used in new drug approval (NDA) applications for the following purposes:
- Demonstrating the validity of models against ICH data
- Bridging clinical-to-commercial changes
- Justifying specification limit, formulation, or process changes
- Selecting commercial packaging
- Defining critical quality attributes
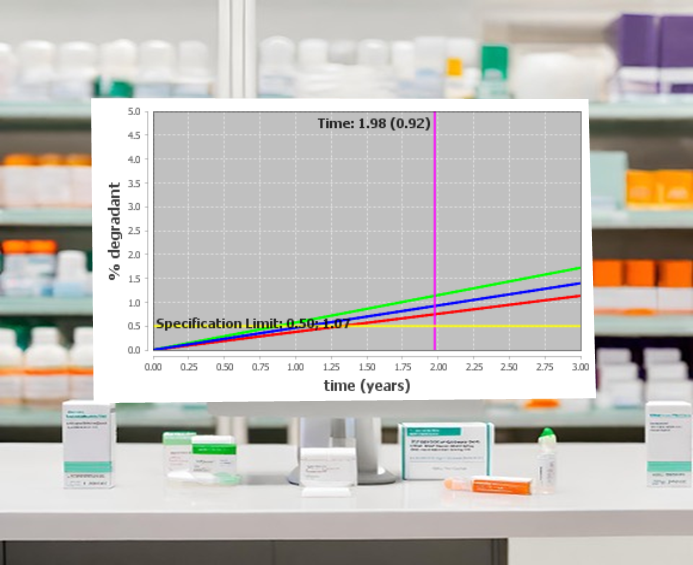
Post-approval applications include justification for reduced-protection packaging and acceptance of after-shipping excursions.
Helpful Resources