Find your missing element with
Thermo Fisher Scientific
Moving from molecule to medicine requires science, technology, and world-class expertise. It also requires a strategic CDMO partnership—bonded by key elements such as communication, dedication, and above all, trust.
The elements of a strategic CDMO partnership
At Thermo Fisher Scientific, we believe that strategic partnerships fuel success. But we also believe that a strategic partnership is more than just providing end-to-end support and solutions, on time and on budget.
A true partnership is built on a combination of intangible elements that work together to build and establish trust. Trust in your expertise and vision. Trust in your technology. And trust in your partners.
We embed these elements of partnership into every operation, interaction, and step across your unique drug development journey.
Transforming CDMO partnerships through a holistic understanding of quality
Bringing your innovative therapy to market requires working with a CDMO partner that embraces quality as a holistic endeavor affecting every aspect of the development process.
Though, despite its critical importance, quality in pharma and biotech manufacturing is often mischaracterized, whether by conflating it with compliance, describing it as an end-of-the-line activity, or attributing responsibility to a single team within the manufacturing organization.
This whitepaper takes an in-depth look at quality, identifying tools and best practices to drive continuous improvement, strengthen collaboration, and build trust within a CDMO partnership.

Our industry-leading capabilities
Whether you have a small molecule, biologic, or an advanced therapy, our flexible and scalable offerings enable you to move from molecule to medicine to market with speed and efficiency.
Insights and Resources
Drug development and manufacturing is an ever-changing industry. Stay connected to new discoveries, innovations, and expert scientific opinions.

Blog
What is a CDMO? Seven things to look for in a quality CDMO partner
Learn how CDMOs (contract development and manufacturing organizations) work with pharma companies, and the top considerations companies have when choosing a CDMO partner.
Read Blog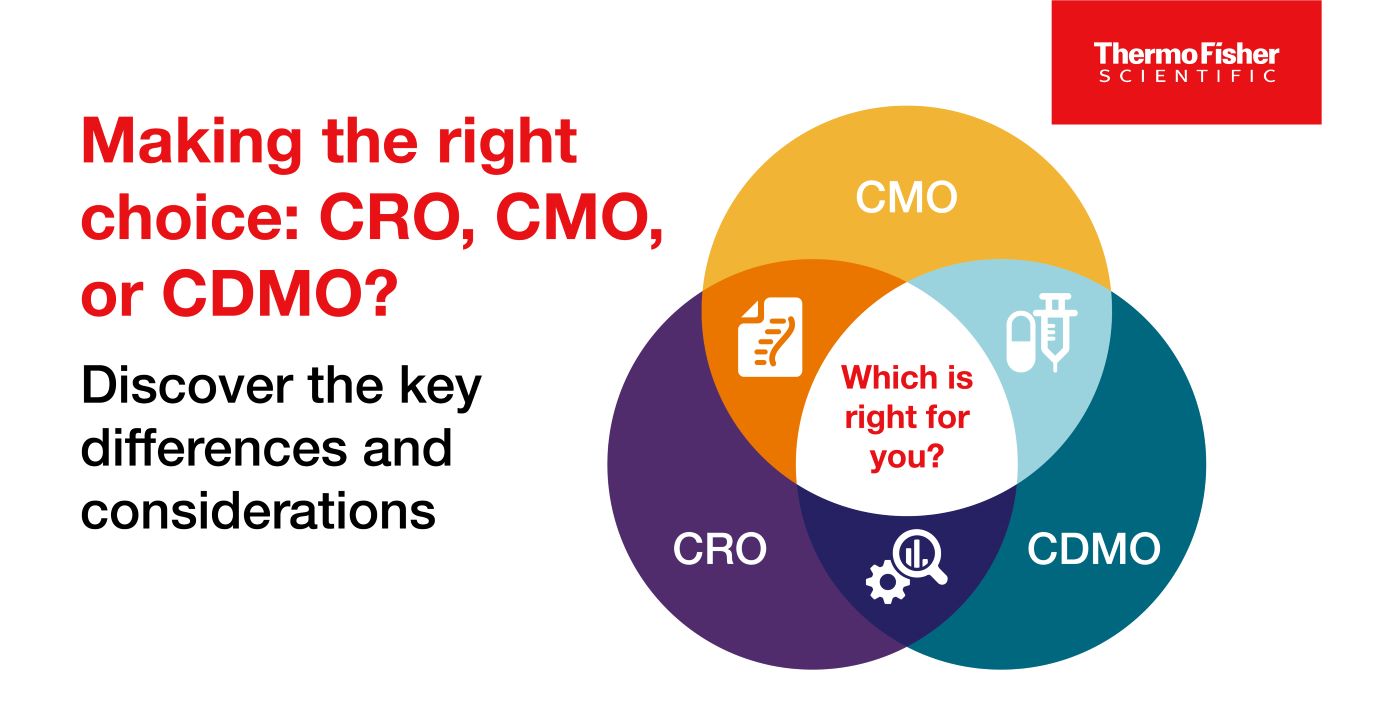
Blog
CROs vs CMOs, and CDMOs: What’s the difference between the three?
CROs, CMOs, and CDMOs all help biotechnology and pharmaceutical companies with drug development and manufacturing, but what’s the difference between the three?
Read Blog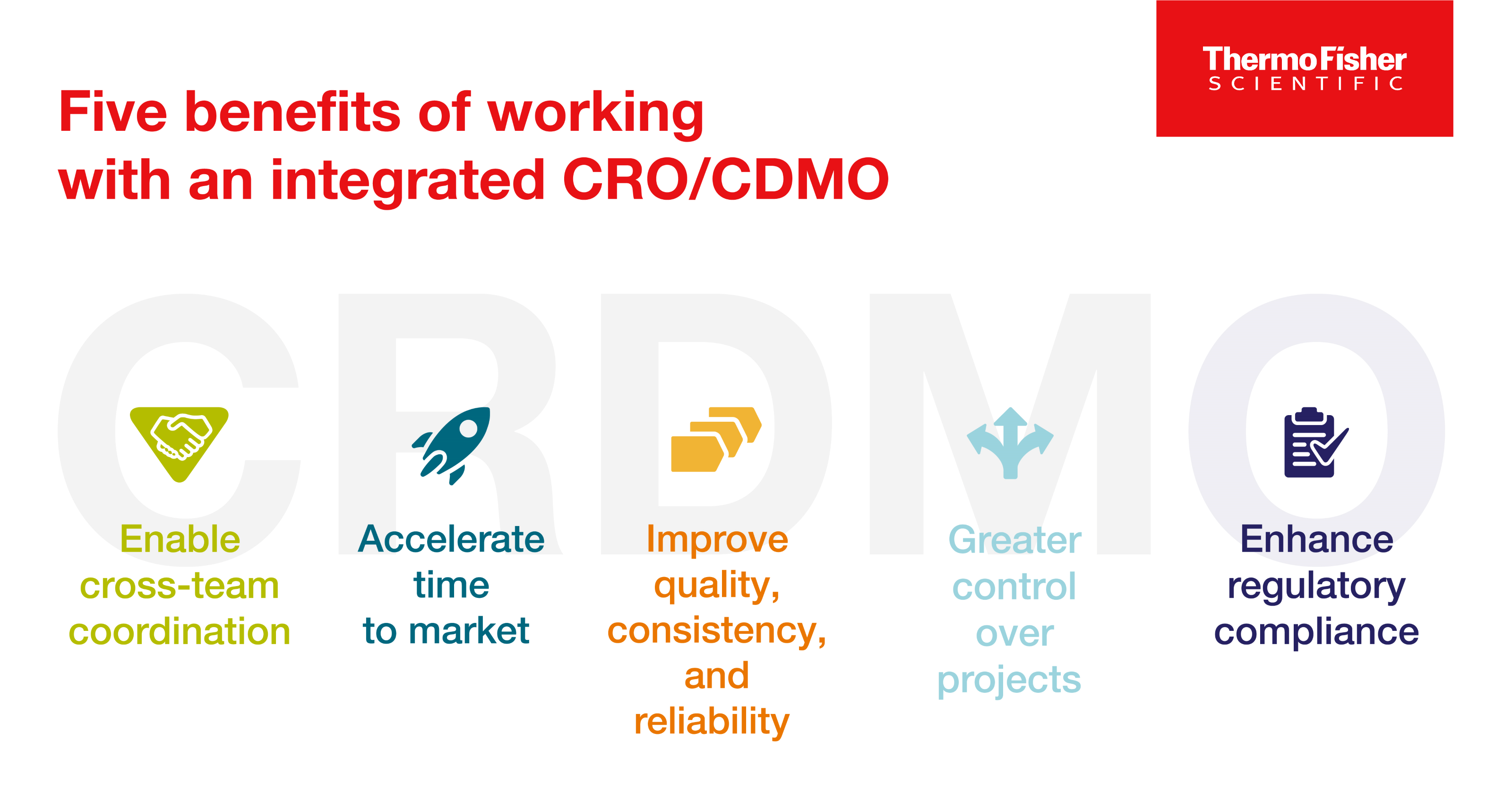
Blog
Exploring CRDMOs: Reshaping research, development, and manufacturing
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
Read Blog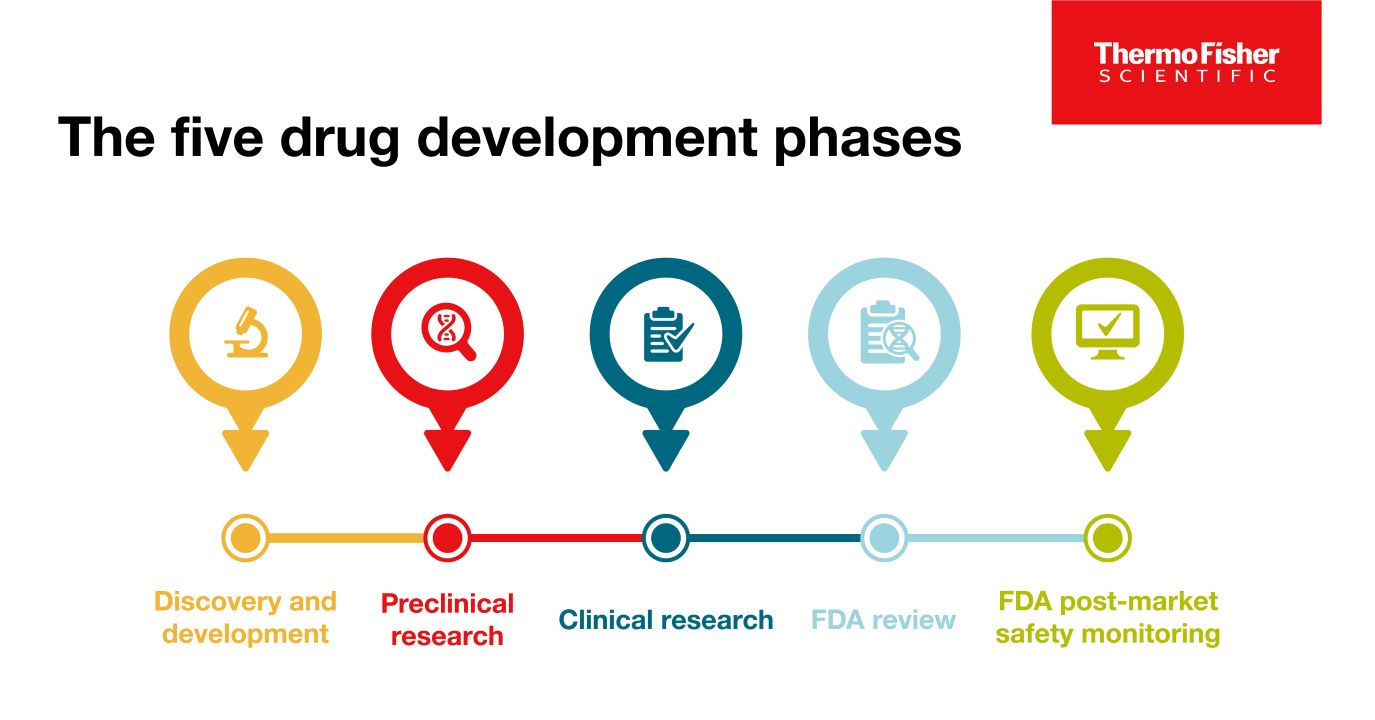
Blog
The 5 Drug Development Phases
To be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) FDA review, and 5) safety monitoring.
Read Blog
Whitepaper
The changing landscape of oncology drug development: Bringing novel lifesaving therapies to patients
Oncology is the fastest-growing, most active sector of drug development. Matching drug products to clinical and commercial needs requires scientific and technological innovation.
Read Whitepaper
Whitepaper
Advancing drug development using in silico modeling
This report provides a framework for that understanding by outlining some of the processes that stand to gain the most from computational modeling and identifying the in silico capabilities that can be used to accelerate and de-risk each phase of development.
Read Whitepaper
Infographic
Preparing biologics for commercialization: Understanding Strategies to Reduce Risk and Optimize Outcomes in Drug Development
Within the drug development process, there are several steps that occur between the laboratory and final manufacture of the drug product. Different players step in during each point, so keeping a program with many moving parts on track requires planning and time-tested execution approaches.
View Infographic
Blog
Enabling a digital culture through integrated business processes
In contrast to a physical work environment, where stability and experience are key, a digital business environment focuses on innovation and connectivity. Learn more.

Infographic
CDMO Checklist to Launch Your Molecule Globally
Preparing to take your drug into the global market? You’ll need to make sure your CDMO has what it takes to successfully navigate the global regulatory space with speed, security, and supply safeguards. Use this quick list as a reference when evaluating your options.
View Infographic
eBook
Protecting tomorrow: Supporting sustainability in the pharmaceutical and biotech industries
Learn how Thermo Fisher is meeting its environmental sustainability goals, and how we work in partnership with the pharma and biotech communities on shared environmental sustainability goals.
View eBook