Adeno-associated virus (AAV) production services
Your trusted partner in AAV development and manufacturing
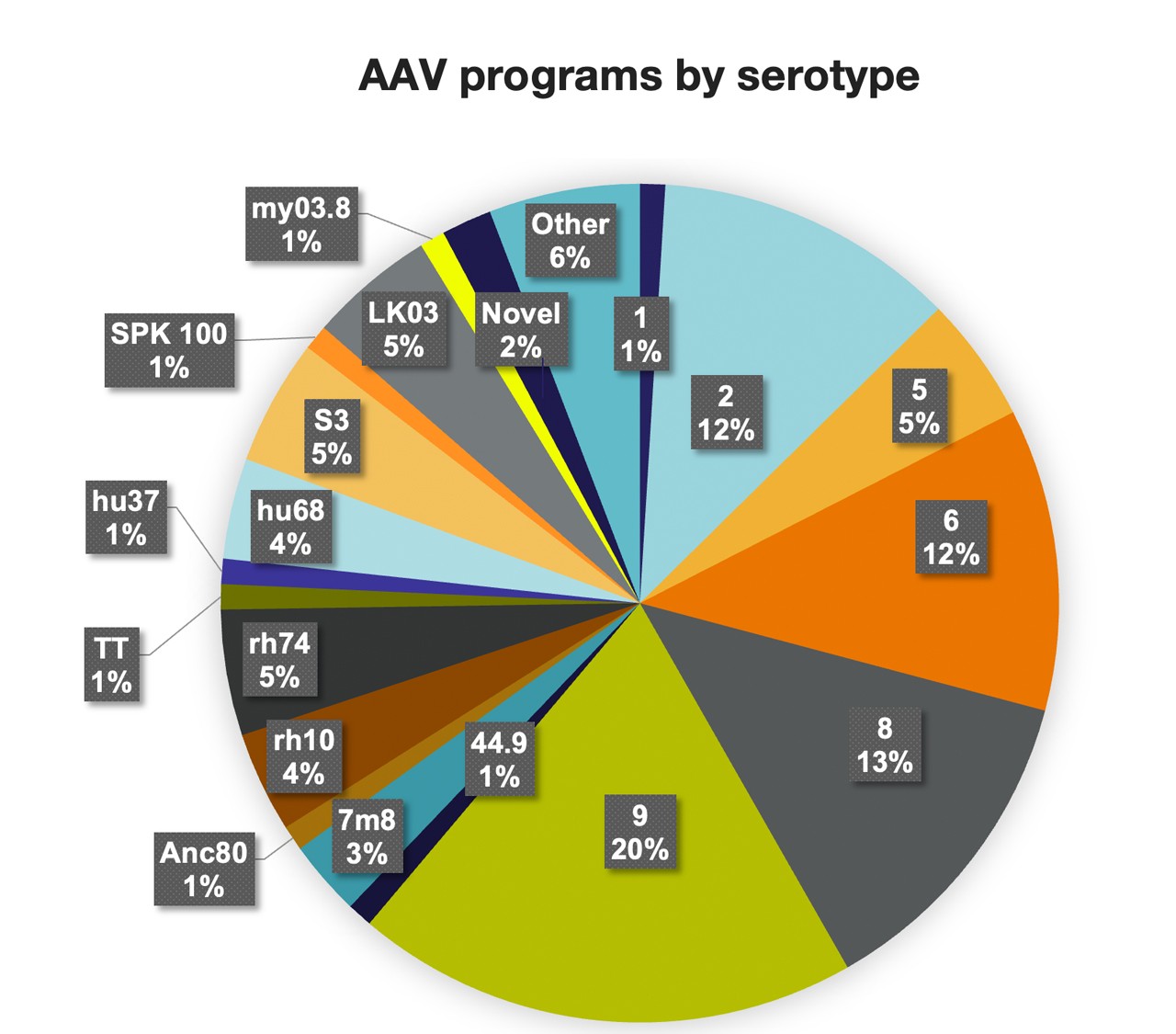
With over two decades of experience, we are industry leaders specializing in a comprehensive array of capabilities for AAV vectors across multiple serotypes.
We’ve manufactured AAV for a total of:
375+ total batches
225+ clinical batches
65+ commercial/PPQ batches
Thermo Fisher Scientific prides itself on cutting-edge capabilities and expertise in both natural and novel serotypes, ensuring the seamless production of AAV vectors. To meet the unique needs of our clients, we employ state-of-the-art technologies using triple transfection in both adherent and suspension cell cultures (mammalian and insect cell lines) as well as producer cell line based viral production and cell line cloning.
- Quality assurance: Quality is the cornerstone of our operations. Our rigorous quality assurance measures ensure the production of AAV vectors that meet and exceed regulatory standards, providing you with the utmost confidence in the reliability of our services.
- Collaboration opportunities: We believe in the power of collaboration. Thermo Fisher Scientific is eager to explore partnerships and collaborations to drive innovation and advance the field of gene therapy. Let's work together to bring your vision to fruition.
- Team expertise: Our team consists of dedicated professionals with diverse expertise in AAV development and manufacturing. From seasoned researchers to skilled technicians, we bring a collective wealth of knowledge to ensure the success of your AAV program.
Our team is experienced in supporting a variety of other viral platforms. For questions about our additional capabilities and capacity, please contact us.
Helpful Resources

