Viral vector development and manufacturing services
Unparalleled experience manufacturing viral vector products for more than 20 years
Viral vector production employs complex processes resulting in varying challenges throughout the product lifecycle. The main issues revolve around selecting a production system, optimizing product quality, and building standardization to enable a robust CMC approach.
Thermo Fisher Scientific viral vector services (VVS) is a leading CDMO that offers a full range of services for the development and commercialization of viral vectors and gene therapy-based vaccines. Our viral vector end-to-end capabilities include process and analytical development, clinical and commercial manufacturing, and fill-finish services. With an extensive network of production sites, global clinical supply chain capabilities, and in-depth viral vector technical and regulatory expertise, we can help you de-risk and expedite your therapy's path to market.
20+ years viral vector experience
Development and manufacturing
3 commercially approved products
and several others pending
900+ lots manufactured
900+ viral vector lots manufactured, including GMP clinical and commercial lots over time
50+ drug substance suites
and 12 drug product suites
The expertise and experience to design a path from early discovery to commercial manufacturing
In addition to core viral vector manufacturing services, we also provide upstream and downstream offerings to support every aspect of your drug development and manufacturing journey. From early translational services to help optimize your small-scale processes to cold chain logistics to facilitate the secure storage and distribution of your final product, we provide end-to-end solutions for managing unique gene therapy and gene-modified cell therapy projects.
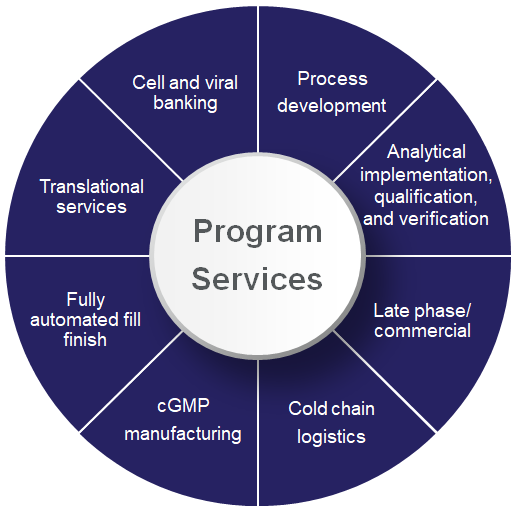
Viral vector types and platforms
While the viral vector market is dominated by adeno-associated viruses (AAV) and lentiviral vectors (LV), there is also a range of less commonly used vectors. Our suspension and adherent processes can be tailored to fit your product, indication, and delivery route based on our diverse expertise in viral vector production. Regardless of your vector strategy, you can be confident that we have the experience and resources to smoothly manage any project.
Specializing in adeno-associated virus (AAV) vectors across various serotypes, we excel in triple transfection and infection techniques for both adherent and suspension cell cultures. Our commitment to excellence ensures efficient and reliable AAV production, meeting the rigorous standards of pre-clinical, clinical, and commercial applications.
We bring extensive experience in process development and GMP production of lentiviral vectors (LV), utilizing both transient transfection and stable packaging cell line-based processes on adherent and suspension platforms. Our expertise has successfully led to the commercialization of products, including a CAR-T therapy, using these LV processes.
Other vector types
In addition to AAV and LV, we also have experience with the following viral vector types leveraging a variety of production platforms.
- Adenoviral
- Retrovial (RV)
- Herpesviral (HSV)
- Modified Vaccinia Ankara (MVA)
- Vesicular Stomatitis Virus (VSV)
- Virus-Like Particles (VLP)
Viral vector CDMO capabilities from discovery to commercialization
From process development and process characterization to manufacturing, QC, and fill finish, VVS has the broad capabilities and expertise needed to develop and manufacture your viral vector product.
With over 20 years of experience creating viral vector products, we have established strategies for upstream and downstream process development and optimization, process scale up, and at scale pilot run standards. Throughout your product life cycle, you'll be guided through each milestone with checkpoints to ensure you meet all quality standards.
Suspension and adherent modalities |
Chromatography-based purification |
Customization and development |
Mammalian and insect cell culture |
Optimization for increased yield/recovery |
Platform assays |
Design of experiments |
Full capsid separation |
Process development testing support |
Scale up |
Specific purity requirements |
Pre-clinical material testing |

Our dedicated viral vector facilities manufacture cGMP-compliant viral vectors for clinical and commercial applications around the world. The 50 drug substance suites can be used for various production modes and technologies (cell factories, iCELLis, SUBs, etc.). Using semi and fully automated fill lines and a range of prequalified vial and container closure systems, we offer a comprehensive aseptic fill and finish service. Through our global technology transfer strategy, we facilitate the successful transfer and establishment of processes into our facilities from our customers or from internal development and pilot laboratories. Quality control (QC) capabilities are available at each site for in-process, release, and stability testing using approved or validated methods.

The regulatory landscape for advanced therapies is constantly evolving, and what is acceptable today may not be adequate tomorrow. Over 15 years, we have supported customers with global regulatory interactions (US, EU, and Canada), CMC regulations, guidelines, and inspections. In addition to providing document reviews and gap analyses, our regulatory services team can generate documents to reduce the number of intermediaries and filing lead times in preparing regulatory filings.
|
|
|
|
Learn how we can help you to navigate regulatory requirements for cell and gene therapies: download fact sheet.
The successful commercialization of your advanced therapy depends on rigorous technology transfer, robust process validation, and specialized regulatory support. Partnering with an organization capable of scaling alongside you ensures sustained capacity, while integrated services streamline your value chain, minimizing risk and accelerating market entry.
- 8 upcoming and ongoing PPQs across VVS sites
- 8 completed process characterizations
- 350+ commercial DS batches
- 250+ commercial DP Batches
- 3 approvals with major regulatory agencies (FDA, EMA, Japan)
- Manufactured in US and EU
Viral vector service offerings
To provide flexibility and agility for our customers, we offer several different pathways for viral vector development to support various manufacturing strategies, timelines, and customization needs.
Rapid Development Framework™ (open platform)
Accelerated development approach for AAV and LV leveraging pre-established processes and analytics with time to delivery of GMP drug product in as little as 9 months.
Customizable development
Flexible development and process solutions for a variety of viral vector types (AAV, LV, AdV, RV, HSV, MVA, VSV, and others) with time to delivery of GMP drug product in 12+ months.
Client tech transfer
Direct transfer and manufacturing of client process with time to delivery of GMP drug product in 9+ months.
Our viral vector manufacturing sites
A global footprint of over 555,000 square feet is available to develop and manufacture viral vector products. We can find suitable capacity, talent, and technology to ensure a robust product and efficient production processes at multiple sites. We are leveraging the viral vector knowledge and experience from Brammer Bio and Henogen S.A., as well as clinical trial capabilities from Fisher Clinical Services.
State-of-the-art Plainville site (290,000 ft2) designed for end-to-end viral vector services, from process development to commercial manufacturing, with unparalleled capacity and capabilities to meet market demands.
The Seneffe site (34,000 ft2) supports both clinical and commercial manufacturing. This site has over 20 years experience in virus and viral vector manufacturing and has been previously approved by EMA for commercial vaccine production.
Our Cambridge facility (140,000 square feet) performs process characterization, process validation, late-phase, and commercial manufacturing. This site has been manufacturing viral vectors since 2018 and received its first commercial license in Q1/2021.
The Gosselies site (8,300 ft2) specializes in process development, analytical development, early-phase clinical manufacturing, and process characterization for process validation.
Helpful Resources
eBook
Gene therapy solutions from discovery to commercialization
Learn more about Thermo Fisher Scientific's gene therapy solutions and strategies to help bring your gene therapies to market.
View eBook
Blog
A CDMO Partner for every Gene Therapy Manufacturing Stage
Take a closer look at the experiences of NysnoBio and bluebird bio for insight into what companies need in a CDMO partner for every stage of viral vector manufacturing and development.
Fact Sheet
Viral vector development and manufacturing services
Thermo Fisher Scientific provides over 20 years of unparalleled experience in developing and manufacturing viral vector products. We specialize in addressing the complexities of viral vector production for cell and gene therapies, including challenges related to production system selection, product quality optimization, and standardization for a robust CMC approach.

Blog
Viral vector commercialization – Part 1: Tech transfer process for commercial viral vector manufacturing
Learn how tech transfers can help develop and manufacture viral vectors at scale, accelerate vaccine and gene therapy commercialization, and provide expertise.

Blog
Viral vector commercialization – Part 2: Best practices in process validation lifecycle
Learn more about the robust viral vector process validation cycle, which includes various assessments and studies to ensure the safety, efficacy, and quality of viral vectors.
Webinar
Benefits of an integrated approach to gene therapy development and manufacturing
Learn about the development and commercialization of viral vectors for gene therapy, so you can navigate these hurdles and deliver the project in a timely, cost-effective manner.
View Webinar
Article
Strategies to accelerate drug development through harmonization of early and late stage processes
This technical article presents a harmonized and streamlined approach established at Thermo Fisher Scientific for manufacturing AAV and LV vectors for discovery research using technologies and processes mirroring current GMP platforms.
View Article
Webinar
Preparing viral vector productions for commercialization
Gene therapy vectors are rapidly approaching the commercial space so commercial readiness is critical for success. Watch our webinar to learn about our capabilities and approaches for preparing viral vectors for commercialization.
View Webinar
Infographic
Regulatory pathways for CGT and ATMP products
CGT is one of the world's fastest-growing therapeutic areas today. Instead of treating patients for the rest of their lives, these therapies offer them hope of a cure. In this infographic, we will review three tips for achieving regulatory success.
View Infographic
eBook
Cell and gene therapies in the US vs. the EU: Top five areas of differentiation
In this eBook we share the five key differences in the drug development and review process for companies hoping to gain market access through US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval—as well as tips for navigating these differences.


