Biologics analytical and formulation development services
Data-driven results to support you with confidence at every step
Creating robust analytical methods and drug substance/drug product (DS/DP) formulations that successfully meet your therapeutic criteria is essential for the success of your biologic, from demonstrating efficacy to gaining clinical adoption.
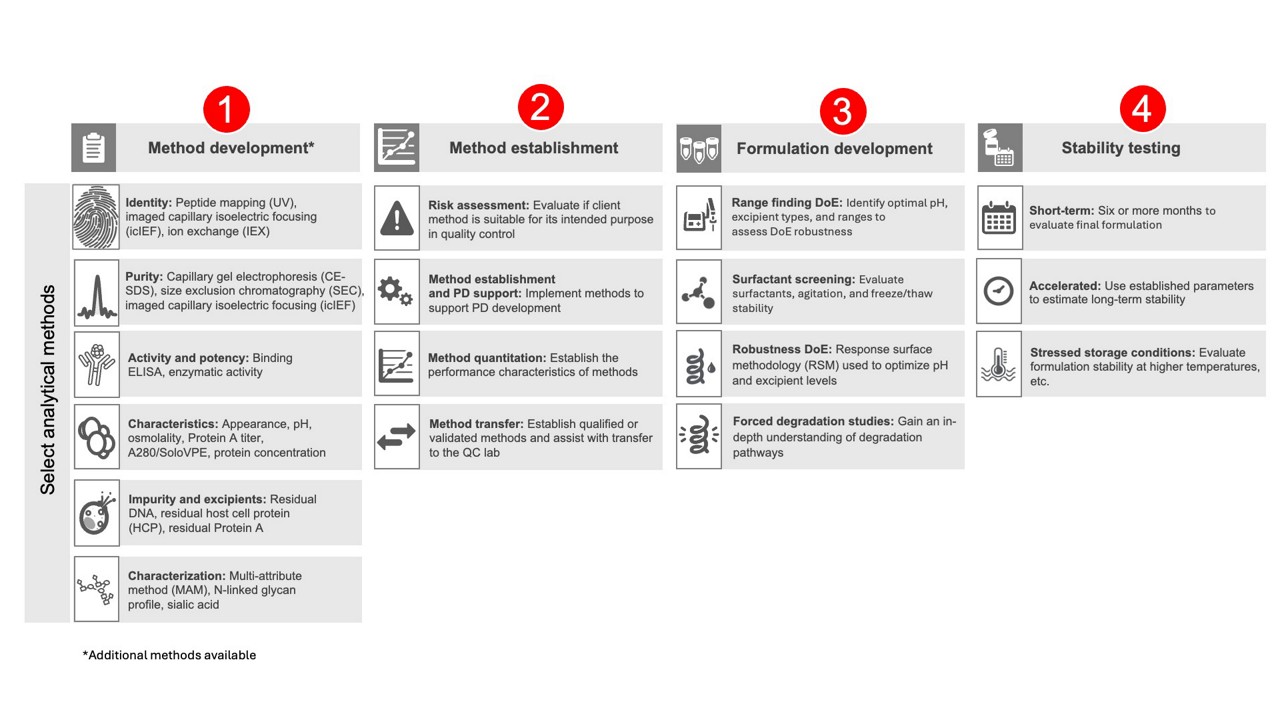
Analytical methods include key tests to verify the identity of the protein or antibody, assess potency, detect any product- or process-related contaminants, and characterize post-translational modifications (PTMs). By incorporating an Analytical Target Profile (ATP) into our enhanced quality attribute monitoring, we ensure that our methods are aligned with your specific therapeutic goals and regulatory requirements, providing a clear framework for your process control strategy.
Equally important in biologic drug substance development is formulation. By developing DS/DP formulations that address patient needs and optimizing key attributes such as ionic strength, pH, shear forces, and surfactants, you can enhance stability, improve storage conditions, and expand delivery options. Product stability is critical, as it confirms shelf life and efficacy over time, ensuring consistent performance and patient safety—both essential in the pharmaceutical industry.
Three key features:
Confidence in product identity
Know what’s in your vials. Among several identity methods, the multi-attribute method (MAM) is used to determine amino acid sequence and identify PTMs such as deamidation, oxidation, glycosylation, and more.
Experienced formulation teams
With industry-leading experience producing hundreds of biologics, our team can help determine the optimal formulation for your molecule, at the concentration your patients need.
De-risk timelines with early stability testing
Stability testing is crucial for determining optimal storage conditions, ensuring biologic drug products are delivered in their intended form, and providing an early read on shelf life to support product release and clinical trials.
Comprehensive Analytical and Formulation Development Capabilities
Meet with our dedicated experts who will work tirelessly to bring your groundbreaking biologic from concept to reality

Elena Gontarz, PhD, St. Louis, Missouri, USA
Holding her PhD in biochemistry with over 10 years of experience in developing biologics for pharma companies, Dr. Gontarz is our resident expert in global analytical and process development, as well as formulation sciences. Currently, Dr. Gontarz is the Head of the Scientific and Technical Affairs team, leading CMC support and biologics programs from Phase I to commercial manufacturing. Meet with Dr. Gontarz to learn more about our analytical method lifecycle development, cell line development, process development, tech transfer, and late phase commercialization.

Palak Patel, St. Louis, Missouri, USA
With over 5 years of experience in early phase biologic development and delivering innovative solutions for pharma companies, Palak is one of our Scientific and Technical Affairs subject matter experts in cell line and cell culture development for biologics. Meet with Palak to uncover how our early phase biologics solutions can help you get your biologic to patients faster.

Otto P.J. Jurrius, Groningen, Netherlands
Holding his master’s degree in biotechnology with over two decades of biopharmaceutical industry experience, Otto is the General Manager at Thermo Fisher Scientific’s biologics manufacturing site in Groningen, Netherlands. Otto leads biologics project management, manufacturing, engineering, and general management across biologics development, as well as clinical and commercial manufacturing. Meet with Otto to learn more about our innovative clinical and commercial manufacturing capabilities.

Erica Byerley, Lengnau, Switzerland
With over 8 years of experience and degrees in both chemical engineering and biological sciences, Erica is our guru in single-use technology, technology transfer, process design, and operational readiness for biologics development through clinical manufacturing. Erica currently leads our global tech transfer engineering team at our brand-new facility in Lengnau, Switzerland. Meet with Erica to learn about our innovative single-use bioreactors or engineering designs that can accelerate your biologics development journey.

Kym Baker, PhD, Brisbane, Australia
Holding her PhD in immuno-vaccine development with over 30 years of experience in developing and manufacturing biologics for pharma companies, Kym has worked on over 400 biologics with 10 through to product commercialization. Currently, Kym leads our award-winning, globally regulatory-approved biologics manufacturing facility in Brisbane, Australia, serving client programs from Phase I to commercial manufacturing. Meet with Kym to learn more about how we can support your products' lifecycle from tech transfer to clinical and late-phase commercialization and manufacturing, or how R&D tax credit and the Clinical Trial Notification (CTN) Scheme in Australia can help accelerate your early phase product to market.
Featured resources

