Mammalian cell line development
Mammalian cell line development services Experts in mammalian cell lines for biologics manufacturing
At Thermo Fisher Scientific, we understand the complexities and challenges that biotech and pharmaceutical companies face when developing biologics. From early-stage development to full-scale clinical commercialization, our comprehensive cell line development (CLD) services are designed to streamline every step of your biologics journey. With over 120 PD programs executed from 2017 to present, we are confident that our dedicated team of experts can help take your program from concept to reality and help to ensure a smooth transition from development to GMP manufacturing with an efficient time-to-market and regulatory compliance.
Technical expertise
We offer expert-driven CLD, leveraging cutting-edge technologies such as AI- and ML-based gene and vector sequence optimization, transposase technology, single cell cloning, and stable clone selection to help ensure your biologic is produced efficiently.
Regulatory and quality prowess
We are committed to providing you peace of mind with our fully cGMP-compliant cell line and comprehensive regulatory support to help ensure your biologic meets stringent quality standards while minimizing regulatory delays during later stage of manufacturing.
Accelerated time-to-market
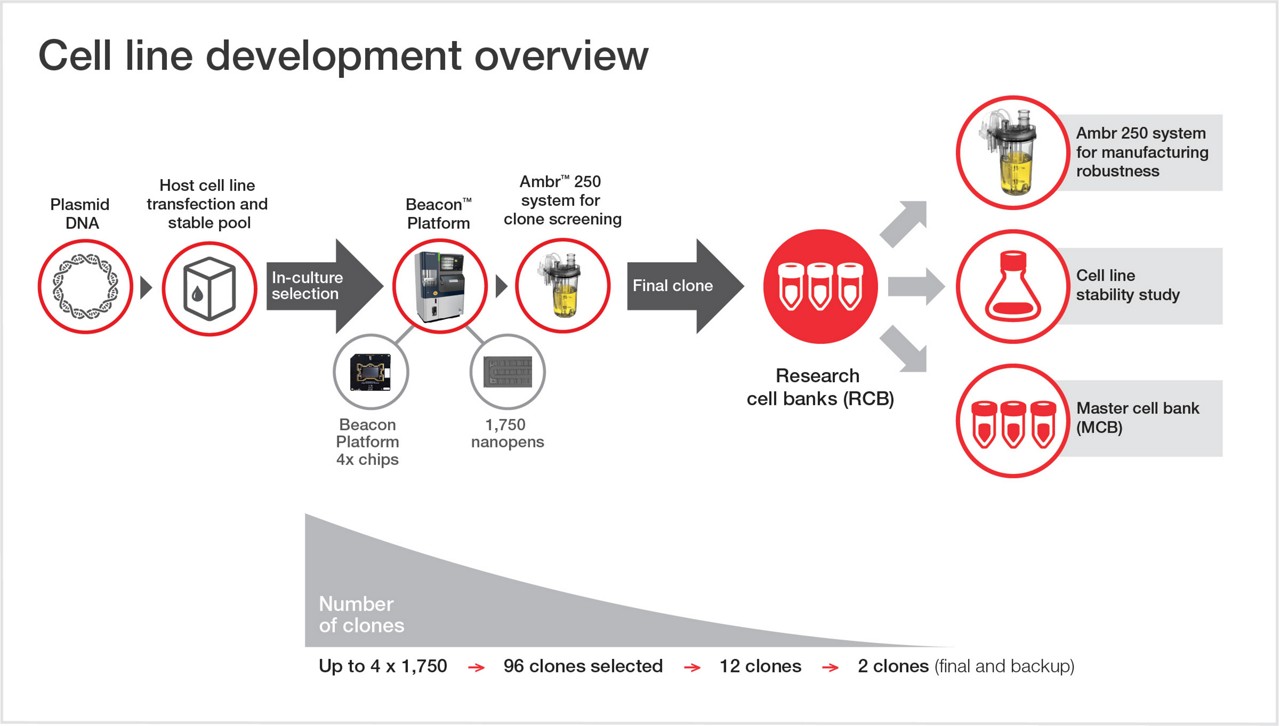
Utilization of high-throughput equipment, including the Beacon™ and Ambr™ 250 system, our robust CLD platform enables rapid cell line generation, to help speed up your biologic’s path from discovery to commercial launch without compromising quality.
Cell line development services
- Increased optimization through AI- and ML-based gene sequence optimization and vector construction
- Transposase-based technology in CHO-K1 cell line helps to enable speed to IND
- Guidance and collaboration throughout your journey from genes to final clone
- State-of-the-art, automated high-throughput equipment including Beacon system, Ambr 250 system, and Tecan™ platforms
- Platform seed train, fed-batch production, and harvest process
- Custom cell line development: use of custom or commercial cell lines, including CHO, myeloma, hybridoma, and PER.C6™ cells
- Simple cell line licensing agreements that avoid royalties or restrictions
- Regulatory guidance and support
- cGMP cell banking services
Featured resources

