Process characterization and validation
De-risk your project with a well-characterized process
Understanding how process parameters affect the quality of your final biologics drug product is critical to the path to commercialization. A clearly defined and well-understood process can prevent expensive missteps, such as batch losses and time-consuming rework, which can result in lengthy manufacturing delays. This knowledge base enables better decision-making around common challenges throughout the product lifecycle and minimizes variability across batches.
Process Characterization (PC) studies are important for several reasons:
- Establishment of the commercial process control strategy
- Definition of product and process specifications
- Enablement of successful process performance qualification (PPQ) and campaigns and regulatory filings
- Support of flexibility and robustness of the commercial process
Our workflows incorporate risk-based and knowledge-based quality by design (QbD) approaches to systematically link process design and control to a product’s critical quality attributes (CQAs)
Our process characterization studies include:
- Unit operation studies, which explore optimization of parameters, performance, and how they change CQAs. Each unit is a part of the manufacturing process, including cell expansion and purification steps.
- Clearance studies for process-related reagents and impurities such as DNA, unwanted chemicals, and host cell contamination; evaluation of methods of detection; and optimizing strategies for removal of impurities and product purification.
- Cell line stability monitors genetic and phenotypic stability and evaluates the cell line’s performance over extended time periods and multiple passages.
- Buffer and media hold studies assess chemical and physical stability across different storage conditions and how they affect product quality.
- In-process biochemical holds focus on the stability of intermediates or in-process materials during the manufacturing process.
- Hold time evaluation to assess the impact of hold times on the stability and quality of in-process materials. This includes biochemical stability and the development of strategies to minimize the impact of hold times.
Three key features:
High-throughput technologies
High-throughput systems (e.g., Ambr® and Tecan Freedom EVO platforms) are used for design space screening to systematically identify key/critical performance parameters.
Small- and pilot-scale models
Scaled-down models are built to represent the commercial manufacturing process including fed-batch, perfusion, and n-1 perfusions processes. Dedicated pilot lab space for both upstream and downstream operations is available.
Automated characterization workflows
PC studies are conducted efficiently by utilizing automated workflows. Examples include automated and in-line cell count and metabolite measurement, recipe-guided continuous feeding regimes, automated buffer preparation, and chromatography method queuing.
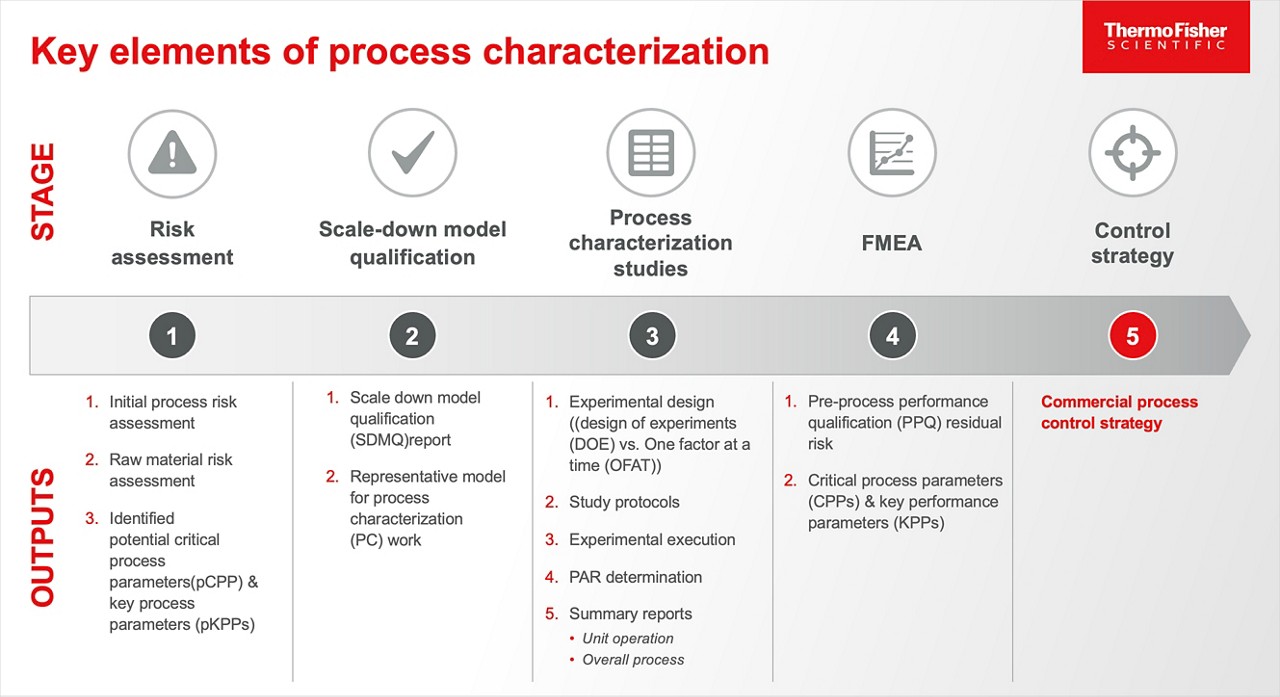
Key elements of process characterization
Helpful resources

