Downstream process development
Providing expertise in downstream process development for biologics
Downstream process development comes with unique challenges, especially when transitioning from benchtop to GMP production and manufacturing. With over 30 years of experience developing purification techniques for complex molecules, Thermo Fisher Scientific’s end-to-end downstream capabilities, open analytical testing, and seamless technology transfer are designed to minimize risk and overcome these challenges to help accelerate your path to market, while delivering high-yield tailored solutions, without sacrificing quality.
Technical expertise
Our team of experts have specialized knowledge and advanced tools to develop high concentration upstream and downstream formulations, as well as optimize purification steps to help ensure high product quality, purity, and process efficiency.
Robust and reliable platforms
Automated, high-throughput process development utilizing Tecan™ liquid handler with robocolumns, and the ÄKTA™ liquid chromatography system to help ensure precise and reproducible protein purification.
Accelerated time-to-market
Our extensive experience in scaling downstream processes coupled with established methodologies and infrastructure support allow us to navigate potential scale-up challenges, avoid pitfalls, and seamlessly technology transfer from development to manufacturing.
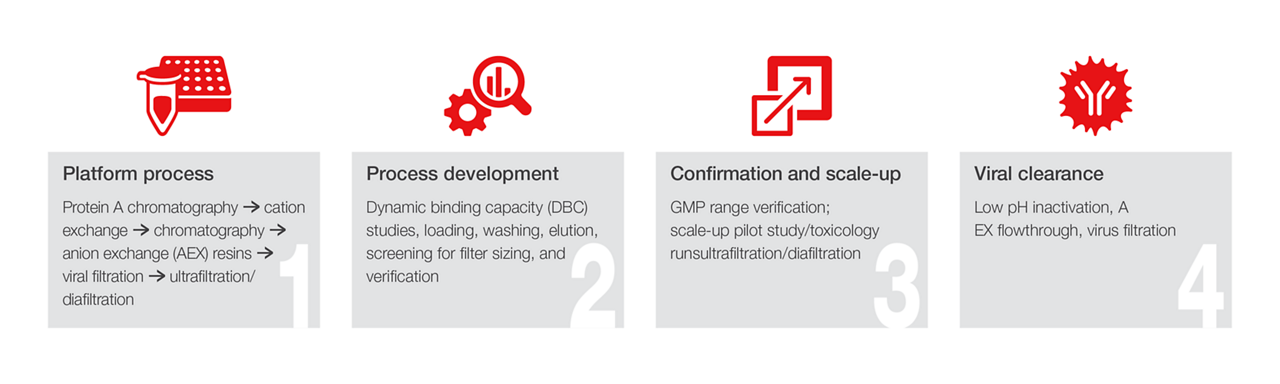
Downstream processing workflow
- Design of experimentation (DoE)
- Process development, transfer, and optimization
- Scale-up from benchtop to commercial scale
- Chromatography—pilot and benchtop scale
- High-throughput screening and filtration
- Open lab analytical testing
- Material generation for studies
- Viral clearance studies
- Process characterization and validation studies
- Process robustness studies
Explore our state-of-the-art global network of biologics manufacturing facilities, where innovation meets precision to produce life-changing therapies
Thermo Fisher Scientific's biologics facility in St. Louis, MO is a center of excellence in single-use bioreactor technology that specializes in mammalian cell development, process development, analytics, and end-to-end cGMP manufacturing services.
Explore our facility in St. Louis, MO
Thermo Fisher Scientific’s site in Brisbane, Australia is a state-of-the-art facility, specializing in clinical and commercial manufacturing services, including clinical cGMP manufacturing.
Explore our facility in Brisbane, Australia
Thermo Fisher Scientific’s site in Groningen, Netherlands specializes in preclinical and commercial cGMP manufacturing and scale-up of mammalian cell culture, recombinant proteins, and monoclonal antibodies.
Explore our site in Groningen, Netherlands
Thermo Fisher Scientific’s facility in Lengnau, Switzerland specializes in highly flexible bioproduction technologies, including single-use bioreactors up to 5,000 L and stainless steel bioreactors up to 12,500 L, providing a pathway from development to large-scale production as your manufacturing needs evolve.
Explore our site in Lengnau, Switzerland
Featured resources

