Upstream process development
Providing expertise in cell culture and upstream process development for biologics
At Thermo Fisher Scientific, we offer a comprehensive suite of upstream process development services to help accelerate your biologics journey from discovery to clinical commercialization without compromising quality, while minimizing risk. With over 30 years of experience across more than 200 molecules, we specialize in the development of monoclonal antibodies (mAbs), bispecific antibodies, Fc fusion, enzymes, recombinant proteins, and many other complex molecules. Our end-to-end upstream capabilities are designed to address your unique challenges, to help accelerate your path to market, and to deliver robust, scalable, and tailored production solutions.
Technical expertise
Our expertise in fed-batch and perfusion culture systems with media screening tools helps to ensure tailored solutions that optimize cell growth, nutrient delivery, and culture conditions to produce any recombinant protein, even with the most challenging characteristics.
High protein yield
Our process development experts leverage advanced bioreactor platforms, customized media formulations, and cutting-edge control strategies to maximize yields while maintaining consistent drug substance quality.
Accelerated time-to-market
With cutting-edge technology, including the Ambr™ 15 and Ambr™ 250 systems, we can maintain precise control over cell culture development and scale-up, maintaining a reliable platform and helping to accelerate timelines.
Upstream process development services
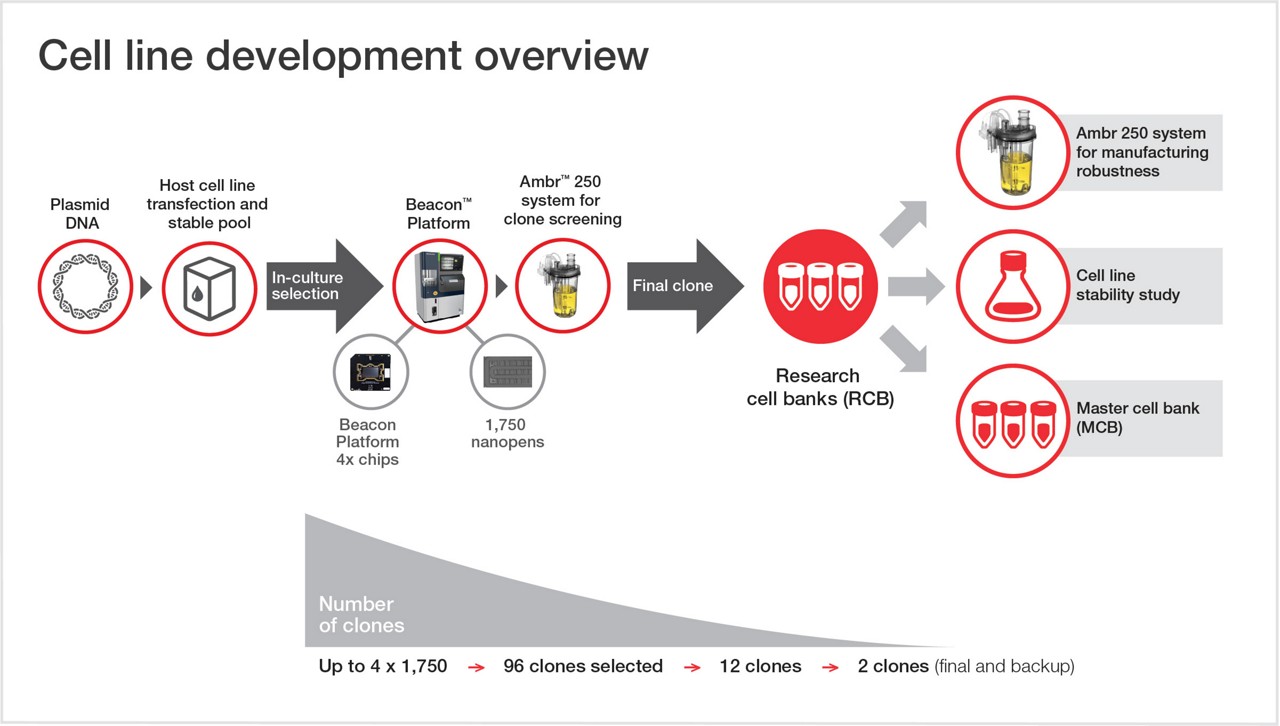
- Clone and media/feed screening, process development/optimization using the Ambr 250 system
- Master cell banking
- Fed-batch, perfusion, and XD™ cell culture process
- Use of high-density mammalian cell culture technologies, including proprietary XD™ technology
- Cell culture process modeling, metabolite, and amino acid analysis
- Single-use scale-up and scale-down models
- Upstream process intensification
- Technology transfer
- Process characterization and validation studies
- Process robustness studies
Explore our state-of-the-art global network of biologics manufacturing facilities, where innovation meets precision to produce life-changing therapies
Thermo Fisher Scientific's biologics facility in St. Louis, MO is a center of excellence in single-use bioreactor technology that specializes in mammalian cell development, process development, analytics, and end-to-end cGMP manufacturing services.
Explore our facility in St. Louis, MO
Thermo Fisher Scientific’s site in Brisbane, Australia is a state-of-the-art facility, specializing in clinical and commercial manufacturing services, including clinical cGMP manufacturing.
Explore our facility in Brisbane, Australia
Thermo Fisher Scientific’s site in Groningen, Netherlands specializes in preclinical and commercial cGMP manufacturing and scale-up of mammalian cell culture, recombinant proteins, and monoclonal antibodies.
Explore our site in Groningen, Netherlands
Thermo Fisher Scientific’s facility in Lengnau, Switzerland specializes in highly flexible bioproduction technologies, including single-use bioreactors up to 5,000 L and stainless steel bioreactors up to 12,500 L, providing a pathway from development to large-scale production as your manufacturing needs evolve.
Explore our site in Lengnau, Switzerland
Featured resources

