Clinical and commercial biologics manufacturing services
Robust and flexible manufacturing solutions for your biologics program
Clinical and commercial manufacturing are crucial final steps in bringing your life-changing biologic product to patients in need. Accurately anticipating and meeting demand can be challenging, even when your process is well-established. An insufficient supply can delay clinical trials and market adoption, while excess production can lead to wasted resources and increased costs.
Whether you begin with us or are looking to expand capacity for an existing commercial process, we can help. Our global technical transfer team—comprising experienced Manufacturing Science and Technology (MSAT) professionals—works closely with clients to build a flexible process grounded in high quality standards, accelerated timelines, and opportunities for cost savings.
Highlights of our technical transfer process:
- Custom facility fit assessment: Using customer-provided information, our MSAT team adapts your procedures to our equipment. They perform hundreds of process assessments each year and often identify opportunities for cost savings.
- Standardization across sites for global manufacturing flexibility: We provide consistent customer support, chemical supplies, and error prevention across sites. Leveraging our global network enables robust supply chains and economies of scale for critical chemicals and consumables.
- Clear communication: We use stage-gate planning and detailed procedures to quickly identify, communicate, and ideally mitigate production delays. We also leverage RACI charts—a project management tool that clarifies roles and responsibilities—to ensure accountability and streamline communication for efficient project execution.
Our facilities utilize single-use technology (S.U.T.)
Minimize contamination risks and enhance production efficiency. Single-use bioreactors (S.U.B.), such as the Thermo Scientific™ DynaDrive™ 5,000 L S.U.B., enable efficient and scalable manufacturing, allowing you to adapt to market demands.
Advantages of single-use technology:
- Reduced labor risk and improved space utilization: A high turndown ratio of 20:1 reduces seed train requirements and increases facility space efficiency.
- Lower upfront and operational costs: Single-use systems reduce cleaning expenses and start-up costs while improving scale-up efficiency.
- Greater flexibility: Quick, easy tech transfer between bioreactors and sites. Thermo Scientific™ DynaDrive™ bioreactors are designed for seamless scale-up, with bioprocessing bags made from the same Thermo Scientific™ Aegis™5-14 film.
Learn how we use single-use technology at our St. Louis, Missouri site. Watch the videos to see S.U.T. in action.
Download our white paper: “The benefits of 5,000 L single-use bioreactors for biologics manufacturing”
We are committed to supporting your manufacturing needs with innovative solutions that ensure efficiency, flexibility, and cost-effectiveness.
Key features of our biologics manufacturing services:
Technical transfer
Our global MSAT team reviews hundreds of processes per year. While developing facility fit assessments, they frequently discover opportunities to save costs while maintaining quality and timelines.
Analytical method development
Several studies are performed to ensure quality and robust processes, including master standard generation, forced degradation, in-use compatibility, and more.
Scale-up manufacture
Sites feature S.U.T., including the Thermo Scientific HyPerforma DynaDrive 5,000 L. With a high turn-down ratio, the S.U.B. reduces manufacturing steps—reducing costs and accelerating timelines.
We provide broad capabilities supporting development, technology transfer, analytical readiness, and cGMP production. Together, these elements help ensure reliable supply continuity throughout the product lifecycle.
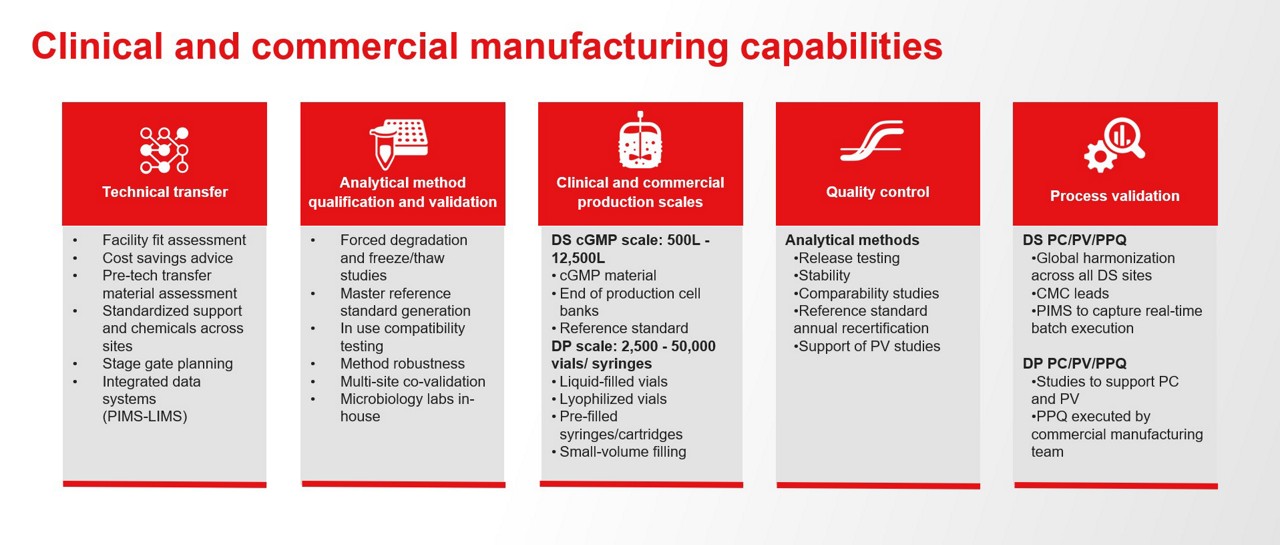
Clinical manufacturing is flexible, small‑scale, and development‑focused, supplying trial materials under phase‑appropriate GMP with limited validation. Commercial manufacturing is large‑scale, fully validated, and tightly controlled, with Process Performance Qualification (PPQ) completed, validated analytical methods and specifications, a mature Quality Management System (QMS), and ongoing continued process verification.
Biologics drug‑substance manufacturing does not include fill‑finish; fill‑finish is the subsequent step. Within our network, specialized sterile fill‑finish sites optimize formulation, perform lyophilization if desired, and package into vials, prefilled syringes, or cartridges. Note that our Path to IND program includes fill‑finish and clinical packaging services.
An effective technology transfer starts with clear, proactive communication. Share comprehensive process and product information before the facility fit assessment so our team can design the most efficient and cost‑effective approach. We also apply stage‑gate planning and detailed procedures to rapidly identify, communicate, and mitigate potential production delays.
Single‑use technology employs sterile, disposable plastic components (e.g., bags) that prevent direct contact with bioreactors, centrifuges, chromatography, and other systems, reducing contamination risk and speeding facility turnaround. While it generates additional plastic waste, its overall environmental impact can be lower than that of traditional stainless‑steel systems due to avoided cleaning chemicals, water consumption, and energy use between runs. The net effect depends on process scale, waste management, and local utilities.
Common analytical methods for biologics include forced degradation (stress by heat, light, oxidation, pH) to establish stability‑indicating methods, freeze-thaw to assess handling limits, and in‑use compatibility with diluents and devices. Core assays cover identity and purity (SDS‑PAGE, CE‑SDS, HPLC/UPLC, LC‑MS), potency (cell‑based or ligand‑binding), charge/size variants (IEX/CEX, cIEF, SEC), glycan/sequence characterization, and residuals/impurities (host cell protein/DNA, endotoxin, bioburden, leachables).
Streamlined seed train with a 20:1 turndown ratio enables direct scale-up from smaller volumes, reducing seed train steps, bioprocessing bag consumption, and labor. Enhanced performance features deliver improved mixing and oxygen mass transfer, supporting robust cell growth and process consistency. Operational efficiency is increased through reduced handling and setup, enabling faster turnaround and lowering the risk of process variability.
All Thermo Fisher biologics sites have in-house regulatory teams and are regularly audited by global health authorities, including the FDA, EMA, PMDA, and EAUA. We maintain a unified compliance and quality assurance framework across regions and have implemented essential master files for key global regulatory agencies.
Featured resources
Learn more about our clinical and commercial manufacturing services:

