The information you provide to the chat will be recorded to improve your experience and to contact you. Please read our privacy notice to see how we are processing and protecting your data. Click to view our Cookie Notice.
We'd love your feedback—take a quick survey to help us improve.
How can we help you today?
Insights and resources
We have internal subject matter experts and work with technical and scientific experts from across the drug manufacturing and clinical trials industry to bring you whitepapers, articles, webinars, and more to help you through every phase of development, from molecule to medicine.
Use the menu buttons to narrow your search, or use the filters below to search the entire library by category, subject matter expert, or content type.
You may be invited to fill out a contact form prior to downloading an asset.
Questions? Contact us

Fact sheet
Clinical ancillary management services for sustainable trials
Explore how we can support your sustainability goals with circular strategies and smart, responsible management of clinical ancillary equipment and supplies.
Fact sheet
Flexible ordering solutions for clinical ancillary management
Discover our customized ordering framework, adapting to protocol complexity, global site needs, and inventory demand to keep clinical trials running smoothly.

Brochure
Scaling biologics with confidence: Proven success from late-stage to commercial
See four real-world case studies showcasing how Thermo Fisher Scientific helps sponsors successfully scale their biologics programs from late-stage development to commercial supply.

Whitepaper
Modernizing Clinical Trial Logistics to Deliver Value
Discover how scalable networks, digital tools, and synchronized processes streamline clinical trial supply and strengthen global reliability and speed.

Blog post
The real challenge of biologics scale-up isn’t capacity—it’s demand
Most biologics never reach the production volumes that justify large-scale stainless-steel capacity. The real challenge isn’t building enough—it’s knowing how much will truly be needed, and when. Here’s why flexibility in scaling is becoming one of biologics manufacturing’s most strategic advantages.
16 minute read

Whitepaper
Early-phase Injectable Formulation Development Strategies
Discover expert best practices for formulation development, including tools like DOE modeling, analytical characterization, and risk-based approaches.

Infographic
Dedicated to development: Bend, Oregon’s impact
Our Bend facility is a center of excellence for solubility enhancement, advanced drug delivery, and early development. Download our infographic to see the site’s impact by the numbers.
Infographic
Path to IND for biologics: Go from DNA to drug product in as few as 9 months
See how Path to IND for biologics can save time while maintaining quality. Learn the key steps that compress timelines, including the use of our CHO K1 cell line with transposase technology.

Blog post
How to Achieve Late-Phase Success in OSD Development
Learn how to achieve late-phase success in oral solid dose scale-up with data-driven strategies, risk mitigation, and the right CDMO partnership.
11 minute read

Webinar
Delivering what matters: Rethinking clinical trial logistics for a global stage
This on-demand webinar explores how biopharma companies can leverage our global transportation and logistics network to design efficient strategies early in clinical trial planning.

Packaging and labeling support for a global cell therapy program
Read how Thermo Fisher Scientific helped a late-stage cell therapy developer execute complex, multi-temperature packaging and labeling for global clinical trials, ensuring on-time delivery and smooth expansion into new regions.

Fact sheet
Importer of Record (IOR) Services
Learn how despite the substantial progress being made toward global regulatory alignment, customs requirements continue to evolve and may differ significantly amongst even neighboring countries.

Webinar
Accelerate with confidence: Research-based insights to guide CDMO–CRO partnership decisions
This webinar shares key findings from a Tufts study that quantifies both the financial returns and risk-adjusted value of working with an integrated CDMO, CRO, and clinical supply partner.

Article
Lipid formulation development: Why softgels are the technology of choice
This article explores why softgels are the technology of choice for today’s biotech and pharma sponsors—and why early development is the best time to address bioavailability issues head-on.

Blog post
CPHI Frankfurt: A leader’s perspective on how strategic integration drives progress in drug development
Integration, digital enablement, and quality are shaping the next phase of biopharma partnerships. In a presentation at CPHI Frankfurt 2025, Jennifer Cannon, President of Commercial Operations at Thermo Fisher Scientific, challenged the audience to rethink how value is created in today’s high-pressure biotech environment, as companies face mounting investor expectations, tighter funding cycles, and growing regulatory complexity.
10 minute read

Infographic
Unlocking ROI and Efficiency in Drug Development
A data-driven look at an integrated approach to drug development.

Webinar
Scaling smart: Overcoming the top roadblocks in biologic drug manufacturing
This on-demand webinar explores how a unified, global CDMO model can help biologic drug developers overcome the most pressing challenges in clinical and commercial manufacturing.

Article
Regional Clinical Logistics Strategy for Success in Europe
Discover how a regional logistics approach can accelerate clinical trials and entry into Europe in an era of geopolitical pressures and shifting trade policies.
Whitepaper
Key Considerations for Labeling Your Cell or Gene Therapy
Learn ways to safeguard products using viable packaging and labeling strategies for your cell or gene therapies.

Blog post
CDMO Supply Chain Excellence with Visibility & Transparency
Discover why real-time data, operational visibility, and transparency throughout the supply chain are essential for operational CDMO excellence and client satisfaction.
10 minute read

Blog post
From promise to patients: Smarter pathways to scale in cell and gene therapy
Cell and gene therapy’s future depends not only on scientific breakthroughs but on how effectively they are scaled. Progress will come from rethinking traditional models—combining flexibility, standardization, and collaboration to move innovative therapies from promise to patient impact. Read the blog to explore how new approaches are reshaping the path from discovery to delivery.
12 minute read

Webinar
Injectable formulation development: Technical strategies for early-phase programs
This webinar discusses formulation development approaches for injectable therapeutics in both liquid and lyophilized formats and shares practical insights on addressing common hurdles.

Blog post
Avoiding regulatory setbacks in OSD development: Why early formulation decisions matter
Early formulation choices can shape the entire trajectory of an oral solid dose program. Missteps in areas like bioavailability, stability, or regulatory documentation often surface downstream, creating costly delays. Aligning formulation strategy with scalability and compliance from the start helps ensure readiness from IND to NDA.
10 minute read

Poster
Advanced Ultra-low Temperature Solutions
Learn more about our advanced technology and meticulous handling practices to provide optimal storage conditions at –80°C and –180°C.

Blog post
Early-stage biotech: The wrong outsourcing strategy costs more than time
For early-stage biotech companies, outsourcing decisions can make or break program momentum. A strategically aligned development model that connects early planning with downstream execution is critical to avoiding delays, reducing risk, and building long-term value.
10 minute read

Blog post
Expert perspective: Navigating regulatory challenges in ancillary supply management
Explore expert perspective on what regulatory challenges are faced in ancillary management, and how to best minimize risk and adapt to evolving requirements.
15 minute read

Video
ATLAS℠: Clinical Trial Label Translations in days
Simplify the complexities of clinical label translations and regulatory approvals, with efficiency, speed, and confidence.

Case Study
Unlocking significant IGST savings and accelerating clinical supply timelines in India through SEZ operations
Read this case study on how Thermo Fisher Scientific helped a client meet aggressive FPI timelines while achieving substantial cost savings through operations in Special Economic Zones (SEZ) in India.

Webinar
Lipid formulations in softgels: Enhancing bioavailability and therapeutic efficacy
This webinar explores how lipid formulations can enhance drug absorption and bioavailability and also examines the various types of lipid systems and their applications across therapeutic areas.
Brochure
PDS Steriles Capabilities: US- & EU-based facilities
State-of-the-art steriles PDS services

Infographic
10 reasons steriles manufacturing isn’t getting any easier
Download our infographic to uncover 10 key challenges in steriles development and manufacturing and see how an experienced, end-to-end CDMO partner like Thermo Fisher Scientific can help.
Case Study
Internal validation study: Ultra-low temperature labels
With many new drugs requiring storage in ultra-cold temperature conditions as low as –80°C, label adhesives can fail from lifting and detachment of labels. Due to a lack of qualified suppliers in the market, learn how Thermo Fisher Scientific validated its own ultra-low temperature labels in various formats. Read the case study to learn more.

Infographic
Autoinjector, pen, and prefilled syringe capabilities
Download this interactive infographic to explore how our scale- and platform-flexible autoinjector, pen, and prefilled syringe capabilities can accelerate your biologic drug development supply chain.

Video
Comprehensive cold chain clinical supply solutions
Explore Thermo Fisher Scientific’s comprehensive, end-to-end cold chain clinical supply solutions that keep your temperature-sensitive materials safe, effective, and delivered on schedule.
Webinar
Accelerating biologics: From final DNA to Phase I in under 9 months
Originally presented at BIO International 2025, this webinar explores how Path to IND helps biotech companies move from DNA to first-in-human Phase I clinical trials in as little as nine months.*
Infographic
India customs warehouse
Thermo Fisher Scientific’s India customs warehouse offers valuable benefits for customers looking to suspend or defer import duties and IGST on materials moving in and out of India. Download this infographic to explore how the Ahmedabad, India site supports global clinical supply chains.

Blog post
Understanding the essential role of Qualified Persons in clinical trials
Navigating complex supply chains while adhering to regulatory compliance in clinical trials can be a challenge. With differences between the UK and EU certifications, Qualified Persons (QPs) play a crucial role in ensuring the highest quality and safety standards. Read this blog post to learn how a strong QP partnership is often necessary for investigational products to reach patients on schedule.
11 minute read
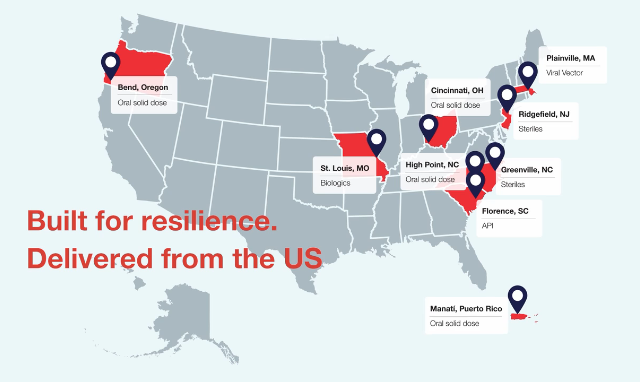
Video
U.S. Investment: Global Complexity Demands Local Resilience
See how Thermo Fisher Scientific is making investments in our U.S. manufacturing network to help customers meet the challenges of shifting global trade dynamics.
Webinar
Cell therapy clinical translation: ensuring speed to clinic while mitigating risk
Position your early cell therapy program for clinical success.
Know the challenges of translating cell therapy products from discovery to clinical manufacturing, and what you can do to address them.

Blog post
Is your API manufacturable—or just technically feasible?
Treating manufacturability as a late-stage concern can lead to costly delays, rework, or even failure. This post explores why early investment in process design is critical—and what manufacturability looks like in practice at Thermo Fisher Scientific’s API center of excellence in Cork, Ireland.
12 minute read

Blog post
Expert perspective: Advancing ultra-low temperature labels for advanced therapies and vaccines
Navigating the complexities of ultra-low temperature labeling for advanced therapies and vaccines is a challenging endeavor. The COVID-19 pandemic has highlighted the need for reliable clinical labeling solutions that can withstand ultra-low temperatures. Julia Field, Sr. Product Manager at Thermo Fisher Scientific, provides expert insights into their innovative ultra-low temperature labeling offerings, highlighting the importance of these advancements in maintaining the safety and efficiency of the clinical supply chain.
15 minute read

Blog post
Expert perspective: How microdosing in clinical trials is accelerating drug development
Learn from our experts, Jutta Wagner and Christian Rose, on how microdosing in clinical trials is accelerating drug development.
15 minute read

Video
Cold chain packaging and labeling overview video
Thermo Fisher Scientific offers state-of-the-art packaging and labeling services tailored for cold and ultracold products. Watch this video to learn about our comprehensive cold chain packaging and labeling solutions.

Webinar
Speed, security, success: Innovations in cold chain packaging and labeling for clinical trials
In clinical trials, speed matters. And for temperature-sensitive therapies, managing cold chain logistics & labeling complexities can be the difference between on-time dosing and costly delays.

Fact sheet
ePly: A digital content distribution platform
Clinical label services introduces ePly, a customized dedicated site linked to a protocol-specific QR code on the clinical label providing access to supplemental information and increased accessibility for improved patient experience and compliance. Download the fact sheet to learn more.

Video
ePLY digital content distribution platform: Overview video
Thermo Fisher Scientific’s new ePLY digital content distribution platform enhances the patient experience by providing easy access to information about investigational medications.
Brochure
Large molecule development and manufacturing: Comprehensive offering enabling speed and flexibility
Remove complexity and bring your large molecule to market faster, with less costs and reduced risks utilizing our integrated and customizable offerings.

Video
Clinical trial kitting services streamline complexity of trial-readiness
Collaboratively designed and expertly assembled kits are tailored to your protocol and maintained at any required temperature.

Webinar
Beyond development: Using continuous manufacturing to accelerate OSD commercialization
This on-demand webinar explores how continuous manufacturing and Real-Time Release Testing (RTRT) can streamline late-phase development and enable faster, more resilient commercial launch strategies for oral solid dose (OSD) therapies.

Webinar
Stop outsourcing, start partnering: Redefining value in global clinical trial execution
11 studies. 51 countries. One team. Learn how reframing the CDMO as a value-driving partner helped NewAmsterdam Pharma’s lean team achieve remarkable results.

Blog post
Accelerate drug development by embracing an integrated approach
Pharmaceutical companies face significant challenges with rising R&D costs and extended development timelines. Embracing an integrated CDMO and CRO approach that combines drug substance, drug product, clinical manufacturing, clinical research, and clinical supply chain management into one streamlined service can simplify complexity, enhance efficiency, and reduce risks, ultimately accelerating the journey from lab to market.
12 minute read
Fact sheet
Clinical trial labeling services
Our clinical trial labeling services support IMP requirements from ambient to ultracold temperatures and are integrated with our packaging, supply chain, and logistics capabilities.

Blog post
Discussing the Future of Biotech
Explore expert insight into what trends are shaping the biotech industry, including innovations, sustainability, AI, and growth in a dynamic landscape.
10 minute read

Webinar
Delivering Value in Clinical Trial Logistics
Global clinical trial complexity is reshaping logistics. Learn what it takes to stay compliant, agile—and focused on R&D innovation.
Infographic
UK customs warehouse—commercial packaging
Thermo Fisher Scientific’s UK customs warehouse solution offers valuable benefits for customers looking to suspend or defer import duties and value-added tax (VAT) on materials moving in and out of the UK. Download this infographic to explore how the Horsham, UK site supports global clinical supply chains.

Fact sheet
Streamline your mRNA workflow with pTRXi plasmid DNA backbone
Download this fact sheet to learn how our fully customizable pTRXi plasmid DNA backbone can accelerate your mRNA development goals.

Fact sheet
Thermo Fisher Scientific’s Omni Client Warehouse
Learn more about our Omni Client Warehouse platform, designed to provide seamless access to your transactional and performance data.

Case Study
Accelerator™ Drug Development: Streamlining preclinical pathways for a faster transition to First-in-Human trials
Download the case study to see how one biotech company saved $1M and shortened development timelines by 12 months with Thermo Fisher Scientific’s integrated CDMO and CRO solutions.

Case Study
Accelerator™ Drug Development: Integrated governance drives execution across global clinical research program
This case study highlights how one biopharma sponsor leveraged Accelerator™ Drug Development to regain control of its clinical research program and deliver results across their portfolio.

Case Study
Accelerator ™ Drug Development: Collaborative timeline management drives speed and simplicity in global vaccine trial
Learn how Thermo Fisher Scientific’s Accelerator™ Drug Development Framework helped a global biopharma company align timelines, resolve quality agreement discrepancies, and streamline execution of a high-stakes vaccine trial.

Video
What does it take to move sensitive biomaterial through a global network?
Learn about the technologies and protocols required to move biomaterial through the cold chain safely, seamlessly, and in full regulatory compliance.

Webinar
Revolutionizing gene therapy manufacturing: Robotics and machine learning for process intensification
This on-demand webinar highlights the benefits of leveraging machine learning, high-throughput process and analytical development, and perfusion intensification for viral vector production.

Case Study
Significant cycle-time reduction enabled by a single-vendor approach for clinical labeling and packaging
Learn how a client who was undergoing an acquisition and name change partnered with Thermo Fisher Scientific for clinical labeling and packaging services to help meet first patient in (FPI) timelines.
Poster
Surrogate cell line for determination of chimeric antigen receptor binding activity
This poster describes a valuable screening tool for selecting the most effective CAR designs for T cell therapies.
Poster
Efficient non-invasive delivery of RNA for somatic cell reprogramming
This poster successfully demonstrates the generation of iPSCs starting with blood cell types using RNA and a novel delivery platform, expanding the choice of starting cells, reprogramming systems, and delivery methods.
Poster
Establishment of reference standards for vector copy number assay and CAR expression
This poster describes the establishment of reference standards for vector copy number and CAR expression to serve as valuable tools for assay qualification and validation.
Poster
ZTTK-iPSC: A framework for the rapid generation of patient-specific rare disease cell models
This poster describes the first successful iPSC generation of ZTTK cells from a 2-year-old patient, as well as the establishment of optimal single cell cloning and gene editing parameters for future experiments.
Poster
Novel detection method for residual beads in CAR T cell manufacturing using imaging flow cytometry
This poster summarizes a novel imaging flow cytometry–based assay designed to improve quality control and reliability in CAR T product release testing.

Whitepaper
Value reimagined: Unlocking ROI and efficiency in drug development
New research demonstrates how aligning manufacturing, clinical research, and supply services under a single partner can reduce complexity, streamline execution, and unlock significant ROI in drug development.

Infographic
How to Select the Right Viral Vector?
View our comprehensive guide that provides the insights you need to make informed decisions and achieve success in your cell or gene therapy development.

Blog post
Five hidden risks in early-phase OSD formulation development
Early-phase oral solid dose development presents a range of hidden risks that can impact long-term success. From API complexity to scalability and regulatory readiness, thoughtful planning can help mitigate setbacks and keep programs on track.
12 minute read

Webinar
Clinical ancillary management: Reducing risk and improving readiness in global trials
This webinar explores how our clinical ancillary management services support patient-first readiness, reduce supply-related delays, and adapt to the evolving needs of global studies.
Blog post
Streamlining biological sample management: The advantages of onsite sample processing and biorepository integration
Discover how integrating sample processing and biorepository storage in one location can accelerate research, protect sample integrity, and reduce operational costs. This blog post outlines key benefits—from faster turnaround times to enhanced flexibility and access to specialized lab networks.
12 minute read
eBook
Gene therapy solutions from discovery to commercialization
Learn more about Thermo Fisher Scientific's gene therapy solutions and strategies to help bring your gene therapies to market.

Webinar
Leveraging early insights to accelerate the oral drug development cycle
You’re an innovative drug developer—don’t let common challenges get you down. This webinar explores obstacles we frequently encounter in OSD development and strategies to overcome them.
Article
Transforming AAV capsid analysis with single particle analysis using mass photometry
Learn how mass photometry works, key advantages and see accuracy and precision results from a pilot study.

Fact sheet
Global clinical manufacturing services
Learn how our global clinical manufacturing capabilities can expedite your clinical manufacturing needs.
Video
Microdosing Technology for Clinical Trials Overview
Learn how our cutting-edge microdosing technology can minimize waste, reduce analytical efforts, and accelerate trial timelines.

Video
Biorepository and Lab Services Overview
Learn how we safeguard critical biological samples with precision-controlled storage and deliver high-quality lab services to support your therapeutic journey.
Whitepaper
Redefining value in drug development: A New model for success
Traditional outsourcing strategies can no longer keep pace with today’s development demands. This white paper makes the case for a more connected model that consolidates infrastructure, expertise, and oversight to reduce risk, streamline operations, and redefine what success looks like in modern drug development.
Blog post
Rethinking 'Platforms’ in Cell and Gene Therapy Development
Discover why developers are looking beyond predefined platform processes and toward flexible customization, like our Rapid Development Framework.
14 minute read

Blog post
Enabling flexibility and scientific innovation in oral solid dose development
A strategic CDMO partnership enabled a clinical-stage pharmaceutical company to navigate the complexities of early drug development, advancing formulation strategies, adapting to shifting priorities, and ensuring a scalable path to clinical trials.
12 minute read

Video
Omni Client Portal: Track Clinical Trial Performance and Progress
Discover our dynamic dashboards that provide accurate, timely, and comprehensive data across receipts, packaging, distribution, and quality workstreams.

Whitepaper
Redefining OSD Development Through Foresight and Innovation
Examine how technology, global networks, customized services and regulatory expertise work together to overcome challenges of oral drug development.

eBook
Accelerate drug development with innovative 360˚ CDMO and CRO solutions: A comprehensive guide to streamlining drug development and reducing time to market
This eBook outlines solutions to help maximize resources and minimize risks across all phases of drug development, supporting each biotech or pharma company’s unique journey to market.
Case Study
Accelerating clinical supply timelines for an emerging biotech
This case study showcases how one biotech company partnered with Thermo Fisher Scientific to meet their clinical trial supply needs, including packaging, labeling, and regulatory translations.

Blog post
Rethinking biologics storage: Navigating nontraditional temperatures
Learn how the right cold chain storage provider can protect your biologics from nontraditional temperature storage requirements
9 minute read
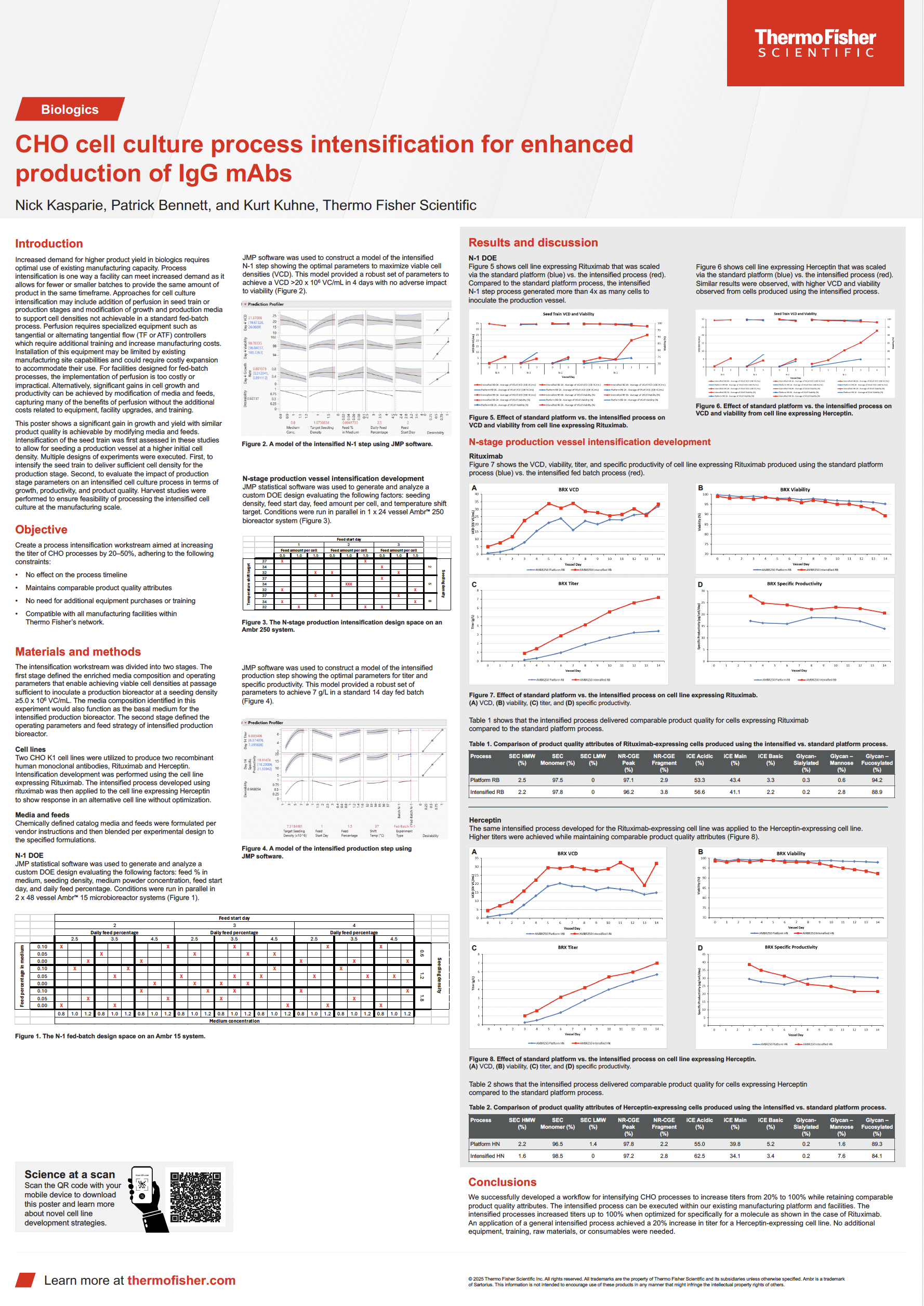
CHO cell culture process intensification for enhanced production of IgG mAbs
Download the case study to see how our scientists leveraged existing manufacturing platforms and facilities with CHO K1 cell lines to increase titer levels by 80% without compromising quality.

Blog post
Decentralized clinical trials: A game changer for biotech drug developers
This blog examines the rise of decentralized clinical trials in the pharmaceutical industry and explores why they’re a game changer for biotechnology drug developers of all shapes and sizes.
10 minute read

Webinar
From model to molecule: Combining AI and experimental strategies to transform drug development
This on-demand webinar explores real-world case studies demonstrating how the integration of computational and experimental strategies can help overcome drug development challenges.
Blog post
Expert perspective: How Thermo Fisher Scientific’s packaging and labeling innovations simplify biotech clinical trials
Find expert insight on how innovative clinical packaging and labeling solutions can streamline processes, enhance efficiency, compliance and overall success.
15 minute read

Fact sheet
Path to IND delivers Phase I clinical trial materials, fast
With Path to IND for biologics, we can deliver your large molecule drug substance for First-in-Human studies in as little as 9 months. Learn more.

Blog post
The science of cell line development for biologics: Improving stability and yield
Cell line development is critical to biologics manufacturing, influencing efficiency, scalability, and product stability. Advances in CHO-K1 cell line engineering, are driving higher yields, improved gene stability, and faster IND submission pathways.
15 minute read

Video
End-to-end support in Bourgoin: From preclinical phases to commercial production
Watch as Pascal Taillardat, technical operations manager, discusses the services in Bourgoin, France—including support from preclinical phases to clinical studies and commercial production.

Blog post
High-potency OSD development solutions: View from Bourgoin.
Explore examples of how biotech and pharma companies leverage our strategically located European facility to solve high-potency API and OSD challenges.
14 minute read

Video
Bourgoin, France: End-to-end support for small biotech and large pharma companies alike
Watch as Anne-Sophie Veck, pharmaceutical development director, discusses our end-to-end drug development services and solutions for small biotech and large pharma companies alike.

Blog post
Advantages of localized Oral Solid Dose production in Europe
Discover how partnering with a localized OSD production facility in Europe can offer advantages, including regulatory expertise and supply chain stability.
15 minute read

eBook
Global reach, biotech speed: Clinical packaging and labeling that delivers
Download this eBook to explore three must-have qualities in a biotechnology packaging and labeling partner and learn how our differentiated solutions can support your next clinical trial.

Video
CDMO excellence at Bourgoin: Advancing OSD drug development
Watch as Isabelle Lafosse-Marin, general manager of our Bourgoin site, discusses our oral drug delivery capabilities—including integrated development, manufacturing, and packaging services.

Video
Inside Bourgoin, France: Our OSD capabilities and the people who make them possible
Watch as Sonia Jourda, operations manager at Thermo Fisher’s center of excellence in Bourgoin, France, shares insights into our OSD capabilities and the people who make them possible.

How Clinical Packaging and Labeling Innovations Drive Value
Effective clinical packaging and labeling drive value by ensuring compliance, protecting product integrity, and improving trial efficiency. A strategic approach helps biotech sponsors mitigate risk, adapt to trial demands, and optimize cost, speed, and reliability.
15 minute read

Blog post
Expert perspective: Navigating the complex path of biologics manufacturing:
The journey from preclinical development to clinical manufacturing in biologics is as intricate as the therapies it aims to deliver. For biopharma companies, understanding the critical steps and considerations of this process can mean the difference between delays and success. Otto Jurrius, General Manager of Thermo Fisher Scientific’s biologics manufacturing facility in Groningen, Netherlands, provides expert insights into the challenges and innovations shaping this complex landscape.
15 minute read

Whitepaper
Overview of hot melt extrusion and its pharmaceutical applications
Our new white paper dives into hot melt extrusion (HME) and its pharmaceutical applications and explores the capabilities of Thermo Fisher Scientific as a CDMO partner for drug development.
Understanding large molecule drugs
This blog provides a deep dive into large molecule drugs, or biologics, exploring their key characteristics, advantages and challenges, and future in the pharmaceutical industry.
18 minute read

Webinar
Securing your samples: Strategies for safeguarding critical biological materials
This webinar explores the significance of ultracold storage and management for biologics and cell and gene therapies, and explains how the right CDMO partner can help safeguard product integrity.

Webinar
Accelerating biologics development with strategies for success
This webinar explores how drug developers can deliver innovative biologics quickly, all while navigating regulatory requirements, clinical trials, production scale-up, and quality control.

Webinar
Opportunities for innovation and flexibility in mRNA and LNP manufacturing
This on-demand webinar explores options within a standard mRNA/LNP manufacturing workflow and highlights innovations to support varying production strategies and enhance your processes.

eBook
Optimizing clinical trial logistics for success
This comprehensive guide discusses the complexities of clinical trial logistics. Learn how Thermo Fisher Scientific’s Total Transportation Management can help ensure timely and secure delivery of clinical trial materials worldwide.

Blog post
The critical role of cold chain logistics: Safeguarding drug integrity from lab to patient
This blog explores the critical role of cold chain logistics in the biopharma industry and explains how a CDMO/CRO partner can help drug developers safeguard the integrity of their products.
(12 minute read)
From clinical to commercial: Streamlining cold chain logistics for advanced therapies
Discover how Thermo Fisher Scientific can optimize cold chain logistics for your advanced cell and gene therapy project.

Optimizing clinical supply management with a one-team approach
Download our case study to explore how our CDMO and CRO services helped NewAmsterdam Pharma deliver investigational medicinal products for over 12,000 patients across 835 clinical sites.

Webinar
Assessing the application of standardized processes in cell and gene therapy development and manufacturing
This webinar features experts in cell and gene therapy (CGT) development, regulatory affairs, and CMC strategy, offering guidance on assessing the risks and rewards of standardized processes.

Blog post
Tamper-evident Packaging for Clinical Supply Protection
Thermo Fisher Scientific’s patented Tamper Evident Carton provides a comprehensive answer to tamper-sealing concerns in clinical supply chains. This design eliminates the need for traditional tamper-evident labels by incorporating the carton, insert, and tamper-evident features into a single, unified package.
(12 minute read)

Video
Benefits of Accelerator™ Drug Development
Explore the benefits biotech and biopharma sponsors can gain through partnering with us through our Accelerator Drug Development’s 360° CDMO and CRO solutions.

Video
Introducing Accelerator™ Drug Development
Learn about our newly launched approach to drug development and how we connect our CDMO and CRO services in a comprehensive and differentiated way.

Video
Comparing Thermo Fisher Scientific to other CDMOs and CROs
Discover how our seamlessly connected global CDMO and CRO services and sites provides an unmatched and unique approach to drug development.

Addressing plasmid DNA challenges in large-scale manufacturing of recombinant adeno-associated virus and lentivirus using enzymatically generated dbDNA
Traditional pDNA manufacturing methods face bottlenecks. This study explores the use of doggybone DNA (dbDNA™), a synthetic alternative to pDNA, for producing rAAV and rLV.

Blog post
Scaling allogeneic cell therapies: Overcoming manufacturing hurdles
Scaling allogeneic cell therapies requires overcoming unique manufacturing hurdles to achieve safe, high-volume production. This article explores key challenges and best practices to maintain quality and efficiency, making these transformative therapies more accessible to patients worldwide.
(15 minute read)

Infographic
Accelerating time to market with integrated CRO/CDMO services
Our new infographic explores the benefits of working with an end-to-end, integrated CRO/CDMO vendor on the complex drug development journey.

Whitepaper
Stabilizing your supply chain in times of global volatility
This whitepaper examines the evolution of supply chain risks over the past 15 years and offers proactive strategies for mitigating vulnerabilities and seizing opportunities for improvement.
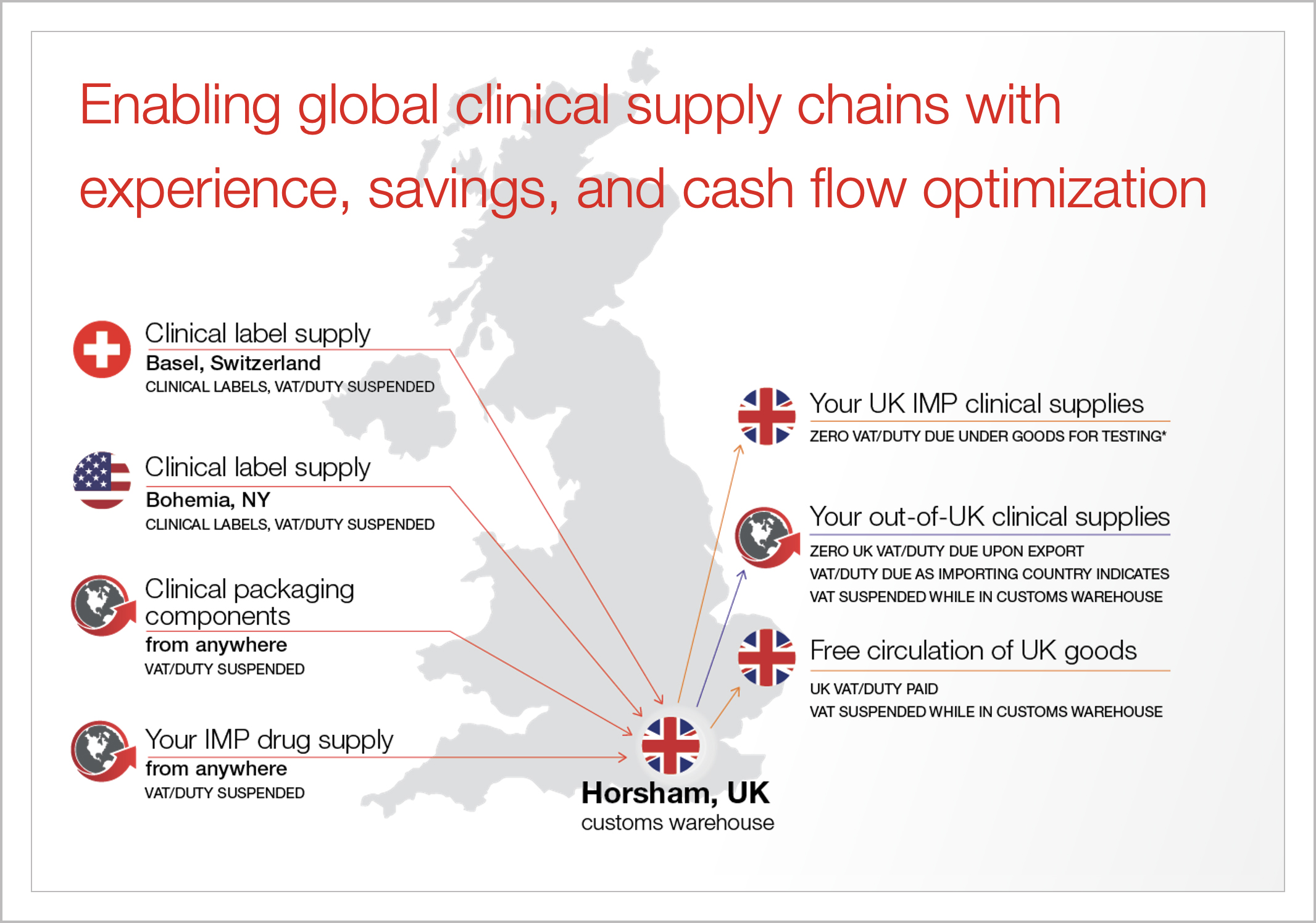
Infographic
UK customs warehouse
Download our infographic to explore how our Horsham, UK, customs warehouse helps global drug developers suspend or defer import duties and value-added tax (VAT) on clinical materials.

Webinar
Maximizing value across drug development: Embracing a new CDMO and CRO partnership approach
Discover how integrating CDMO and CRO services and solutions under one comprehensive model can improve efficiency, reduce timelines, and help deliver high-quality therapies to patients faster.

Webinar
Unlocking the potential: Challenges and opportunities in oral delivery of peptides
Led by Anil Kane, Ph.D., MBA, this on-demand webinar explores the key challenges of oral peptide delivery, along with innovative solutions and future opportunities in pharmaceutical development.

Blog post
Redefining acceleration of the drug development journey
In the pharmaceutical industry, getting essential treatments to patients quickly and safely demands a comprehensive approach that reduces delay, complexity and risk throughout the drug development journey. Accelerator™ Drug Development, Thermo Fisher Scientific’s 360˚ CDMO and CRO solutions, brings all those necessary services under one roof, enabling customers to achieve their goals of getting treatments to patients faster.
16 minute read

Blog post
What are Small Molecule Drugs?
Explore the ins and outs of small molecule drugs, including key development and manufacturing challenges, as well as their future potential in modern medicine.
16 minute read

Brochure
Advanced therapy services
This “Advanced therapies” brochure outlines our end-to-end CDMO development and manufacturing services and solutions for advanced therapies, including viral vectors, cell therapies, and mRNA.

Blog post
Insights from CPHI 2024: Navigating the high costs of cell and gene therapy development with flexible financial solutions
Cell and gene therapy developers face significant financial challenges that can stall critical projects. Flexible financial solutions are helping companies manage costs and advance their therapies. During CPHI 2024 Milan, Kelly Howard, Vice President of Commercial Operations for Viral Vector, mRNA, and Cell Therapy Services at Thermo Fisher Scientific, discussed flexible options available through Thermo Fisher Financial Solutions.
8 minute read

Blog post
Manufacturing autologous cell therapies: challenges and best practices
Manufacturing autologous cell therapies is a complex and resource-intensive process that involves unique challenges like supply chain complexity, scalability, and high costs. These therapies, which utilize a patient's own cells, require a highly personalized approach at every stage. However, by adopting best practices—such as optimizing supply chain management, implementing scalable manufacturing solutions, and staying aligned with regulatory requirements—manufacturers can enhance efficiency and reduce costs, making these life-saving therapies more accessible to patients in need.
(17 minute read)

Mastering Drug Development Challenges through Tech Transfer
Discover the six components of predictable and efficient tech transfer and four tools and techniques that are shaping our industry-leading approach.

Whitepaper
Sustainable systems: Evaluating the environmental impact of single-use biomanufacturing technology
This whitepaper explores the advantages of sustainability with respect to single-use bioreactors, buffer and media hold containers, centrifuges and other biomanufacturing equipment.

Whitepaper
The benefits of 5,000L single-use bioreactors for biologics manufacturing
This whitepaper outlines how to evaluate capacity needs and the benefits of using single-use bioreactors (SUBs) at different drug development stages in biologics manufacturing.
Technology transfer: Best practices for optimizing success and mitigating risk in sterile drug manufacturing
Discover key strategies and considerations to optimize success and mitigate risks while planning a technology transfer for a parenteral drug product.

Clinical supply optimization services: Project management support model
Download our fact sheet to explore our project management options, including Core, Enhanced, and Enhanced+ service tiers for clinical supply optimization.

Webinar
Optimizing pharmaceutical transportation management in Europe: Mitigating risks in the supply chain
This expert-led webinar explores the intricacies of clinical trial logistics and pharmaceutical transportation management for temperature-sensitive advanced therapies, particularly in the EMEA region.

Blog post
Emerging trends in cell therapy: Autologous and allogeneic perspectives
The cell therapy sector is experiencing significant growth, driven by innovations in biotechnology and increased investment in research and development. Despite the promise of these therapies, the intricate manufacturing processes for both autologous and allogeneic treatments present significant challenges. Thermo Fisher Scientific’s commitment as a manufacturing partner is to stay at the forefront of these advancements, driving innovation and ensuring the highest standards in cell therapy development and manufacturing.
10 minute read

Webinar
Navigating complex supply chains: The critical role of UK and EU qualified persons
This webinar explores the roles of Qualified Persons (QPs) in the UK and EU, highlighting regulatory updates, supply chain continuity, cold product challenges, and strategies for effective collaboration.

Webinar
Enhancing oral drug delivery: Exploring multiparticulate systems
This webinar delves into the intricacies of multiparticulate systems for oral drug delivery, exploring their design, benefits, and the issues they are uniquely positioned to solve.

Brochure
OSD capabilities overview
This brochure details Thermo Fisher Scientific’s global CDMO services and solutions for oral solid dose (OSD) development and manufacturing, so you can know what’s ahead on your journey to market.

Blog post
Understanding cell therapies: Key differences between autologous and allogeneic approaches
By harnessing living cells to repair, replace, or regenerate damaged tissues and organs, cell therapy offers personalized and potentially curative treatment for a wide range of conditions. As the field evolves, understanding the nuances between different types of cell therapies is crucial for professionals involved in the development and manufacturing of these therapies.
12 minute read

Clinical ancillary management: Flexible service tiers
Download the fact sheet to explore Thermo Fisher Scientific’s Clinical Ancillary Management service tiers, which include Basic, Standard, and Premium levels for clinical trials of every shape and size.

Why process development matters: Six benefits for biotech and pharma companies
This blog explores the essential role of process development in drug development and manufacturing, highlighting six key benefits that sponsors can gain by prioritizing this task.
15 minute read

Webinar
Leveraging a flexible and efficient Rapid Development Framework™ to accelerate development and manufacturing of cell and gene therapies
Learn how our Rapid Development Framework™ for processes and analytics can accelerate development and manufacturing timelines while remaining adaptable to the unique needs of each product.

Webinar
Navigating cold chain complexity with innovative solutions
Explore challenges with cold supply chain logistics, and how advanced services can preserve the safety of your products while transporting them to patients in need.
Q&A: Solid-state characterization and crystallization process development
In this article, the subject matter experts of Thermo Fisher Scientific answer frequently asked questions about the importance of solid-state characterization and crystallization process development.
Route scouting for effective early-stage process development
This article explores why early-stage process development, which includes route scouting and solid-state characterization, is critical for developing robust, scalable, and cost-effective manufacturing processes.
Q&A: Route scouting for cost-effective process development
Process research, or route scouting, is crucial for ensuring the scalability and efficiency of drug production. Learn about our expertise in this area by downloading our Q&A with industry-leading experts.
Solid-state & Crystallization Development for Speed to Market
This article explores how drug developers can accelerate product timelines and minimize costly errors by prioritizing solid-state characterization and crystallization development early on.

Video
Green chemistry in the CDMO world: Thermo Fisher Scientific's approach to sustainable API development and manufacturing
This video showcases Thermo Fisher Scientific's dedication to green chemistry in API development and manufacturing, highlighting our leadership in creating a more sustainable future for pharma.

Infographic
9 Ways to Ensure a Reliable Tech Transfer
Download our infographic to explore nine best practices for achieving a successful tech transfer, a vital process in the pharma industry as operations scale or shift.

Infographic
5 CDMO attributes for reliable technology transfers
Download our infographic to explore strategies for ensuring reliable and simple technology transfers and the importance of collaborating with a CDMO that embodies five key attributes.

Webinar
The road to commercial readiness: Mastering API production at every scale
This webinar explores Thermo Fisher Scientific’s holistic approach to API commercial production, which encompasses scalability, sustainability, cost optimization, and manufacturing flexibility.

Embracing green chemistry for sustainable API development and manufacturing
Early-stage API development, despite its inherent risks and uncertainties, is the critical period for embedding green principles. This proactive approach not only reduces environmental impact but also lays the foundation for scalable and cost-effective processes as the compound progresses through clinical phases.
9 minute read

Blog post
The power of partnership in small molecule discovery and development
Pharma companies can streamline their small molecule drug development by leveraging the internal synergies of a single, trusted provider for discovery resources and development and manufacturing services.
9 minute read
The critical role of tech transfer and the value of strategic partnerships
In pharmaceutical manufacturing, tech transfer is the systematic transfer of knowledge, processes, and technologies from the development phase to commercial manufacturing and across production sites. Learn more.

Webinar
Discover the road to success for viral vector production
This webinar explores the critical elements of viral vector production for gene therapies, focusing on the technical and regulatory challenges inherent in this field.
Blog post
Advancing viral vector development through Quality by Design approach
Explore more on NysnoBio's partnership with Thermo Fisher, focusing on a quality by design approach to viral vector development and manufacturing.
12 minute read

Blog post
Meeting biopharma challenges: An insider’s view on CDMO solutions for biologics
Maider Parikh, Ph.D., Vice President, Commercial Operations, Biologics at Thermo Fisher Scientific discusses the key biopharma challenges including meeting project timelines, navigating regulatory requirements, and ensuring supply chain reliability, and explains how advanced technologies, expertise, and strategic planning enable Thermo Fisher to address these challenges and help bring biologic products to market faster.
9 minute read

Video
Mike Pearson’s story: Leveraging an integrated network for IND filing
Learn how the team at our Bend, Oregon site used just-in-time manufacturing to meet a client’s end-of year IND filing schedule.

Video
Jessica & Jigal’s story: Overcoming roadblocks to expedite drug delivery
Explore the story of employees Jessica Morin and Jigal Shah, as they navigate a challenging situation involving the distribution of an antibacterial drug.

Video
Real-time Track and Trace platform
Learn more about our Real-Time Track and Trace platform, a cutting-edge solution designed to provide unparalleled oversight into cold chain supply management.

Tech transfer, part 2: The value of strategic partnerships in technology transfer
Strategic partnerships play a crucial role in successful technology transfers by helping to identify and mitigate process risks. A well-chosen strategic partner can preserve project timelines, overcome common technology transfer challenges, and lead to significant cost savings.
10 minute read

Tech transfer, part 1: Navigating uncertainty in pharmaceutical manufacturing: The critical role of technology transfer
Efficient technology transfer in pharmaceutical manufacturing helps maintain product quality, protect intellectual property, manage costs, and scale operations, thereby ensuring that companies can respond effectively to new opportunities or challenges, maintain competitive advantage, and ensure uninterrupted supply of medications to patients.
15 minute read

Webinar
How to Ensure Reliable and Simple Tech Transfer
Explore the importance of reliable technology transfers for oral solid dose products from development to manufacturing and key enablers in this process.

Whitepaper
Advancing drug development using in silico modeling
This report provides a framework for that understanding by outlining some of the processes that stand to gain the most from computational modeling and identifying the in silico capabilities that can be used to accelerate and de-risk each phase of development.

Pioneering innovation in pharmaceutical lyophilization
Learn more about the lyophilization process, challenges faced in the industry, as well as the growing demand for lyophilization services provided by CDMOs.

Brochure
API capabilities overview
Explore Thermo Fisher Scientific’s CDMO offerings for active pharmaceutical ingredients (APIs) for the development and manufacturing of small molecule drugs.

Webinar
CDMO 2.0: Uncovering the missing element in next-generations pharma partnerships
This webinar explores how CDMO supply chains are transforming into value chains, and discusses the increasing importance of CRO/CDMO collaborations in advancing the pharmaceutical industry.

Video
Katie Shannon’s Story: Colleague Behind Facility Construction
Learn more about Katie and how she’s tailoring Thermo Fisher’s facilities for viral vector production, accommodating diverse sizes and scales of processes and platforms.

Infographic
Bioavailability enhancement technologies for poorly soluble molecules
It’s estimated that about 40% of drugs with market approval and nearly 90% of molecules in the discovery pipeline are poorly soluble. This can impede drug absorption, leading to reduced bioavailability and compromised therapeutic efficacy.
Explore how Thermo Fisher Scientific’s Quadrant 2TM platform leverages AI/ML tools to identify solubility enhancement technologies.

Webinar
CDMO 2.0: Uncovering the missing element in next-generation pharma partnerships
This webinar explores the next generation of CDMO partnerships and discusses how these strategic collaborations are set to transform the pharmaceutical landscape and drug development.

Video
How a Family’s Perseverance Helped Them Reach a Seemingly Unattainable Goal
As members of the United States Air Force, Lauryn and Chris were as healthy and fit as a young couple could be. But in the world of genetics, all it takes is a couple of proteins lining up in the wrong way, and things can change in a hurry.
Article
From early innovations to commercial triumphs: One viral vector partner for every stage of gene therapy development
Our article explores the partnership needs of two companies at opposite ends of the continuum for gene therapy development and how both—NysnoBio and bluebird bio—found the support they were looking for to deliver their innovative therapies to patients.

Video
Pete & Lisa Beerse’s story: Navigating aftermath of an accident
Learn more about Pete’s journey navigating the aftermath of an accident, and how Thermo Fisher Scientific helps develop life-saving drugs for patients like Pete.

Five best practices for integrating drug substance and drug product development
Discover five best practices to consider when integrating drug substance and drug product development to optimize the efficiency and effectiveness of your approach.
9 minute read
Article
Pros of building vs buying a biorepository
This article explores the advantages and disadvantages of building vs. buying a biorepository for cold and ultracold storage needs, and how a CDMO partner can provide support.

Video
Innovation Lab
Watch our video about Thermo Fisher Scientific’s Innovation Lab in Center Valley, Pennsylvania, a state-of-the-art facility that will help advance the future of clinical trials worldwide.

Webinar
Leveraging AI-powered solutions for drug solubility and bioavailability
This webinar explores the potential of artificial intelligence and machine learning in early drug development, particularly in designing solutions for solubility and bioavailability enhancement.

CDMO quality harmonization: Ensuring consistency, reliability, and supply chain resilience
The harmonization of quality standards, procedures, and practices across sites is crucial for enhancing the resilience and efficiency of the entire supply chain and is a key driver in accelerating market entry for safe, effective therapies.
14 minute read
Article
Navigating an uncertain regulatory environment for mRNA-based products
This article outlines best practices for navigating an uncertain regulatory environment for mRNA-based products and explores why partnering with a CDMO can provide peace of mind.

What’s hot in freeze drying? Your lyophilization questions answered
Lyophilization is a critical process in the sterile fill-finish phase of pharmaceutical manufacturing, particularly for products that require high levels of stability and a longer shelf life. The ability to transform drug products into a dry powder without compromising their structural integrity is particularly crucial for preserving the stability and efficacy of biologic products, such as vaccines, antibodies, and other protein-based therapies. Over the years, advancements in technology and process optimization have made lyophilization more efficient and reliable for a wide range of pharmaceutical applications.
15 minute read

Webinar
Reducing the carbon footprint and associated cost of pharmaceutical packaging
By implementing innovative packaging technology and design strategies, pharmaceutical companies can minimize their environmental impact while satisfying evolving laws and regulations. Learn more.

Webinar
Advancing vector-based gene therapies for Parkinson’s disease
This on-demand webinar explores NysnoBio’s groundbreaking journey in developing an adeno-associated virus (AAV)-based gene therapy for Parkin-PD.

Revolutionizing drug development: AI-driven solutions for solubility and bioavailability challenges
The integration of AI/ML technologies in drug development, particularly in addressing solubility and bioavailability challenges, marks a significant paradigm shift in the pharmaceutical industry. These advanced computational methods are transforming the traditional resource-intensive trial-and-error processes into more efficient, accurate, and cost-effective strategies.
12 minute read
Mastering complex small molecule APIs and formulations
Complex small molecule APIs are characterized by their intricate structures, higher molecular weights, and sophisticated delivery requirements, which pose unique challenges in their formulation and manufacturing processes.
15 minute read

Webinar
Positioning early cell therapy programs for clinical success: Insights to mitigate risk and ensure GMP readiness
Explore key considerations for early-phase cell therapy developers, including evolving regulatory guidelines and common manufacturing issues. Additionally, learn about orchestrating a successful cell therapy clinical trial.
Whitepaper
Optimizing the cell and gene therapy patient journey through integrated CRO/CDMO partnership
Cell and gene therapies have the potential to fundamentally change treatment paradigms for patients living with a wide range of diseases, including genetic disorders, rare cancers, and neurological conditions.
Predictive modeling for solubility and bioavailability enhancement
Explore the challenges faced by poor solubility and low bioavailability in pharmaceutical formulation and the potential of predictive modeling to overcome these challenges.
15 minute read

Fact sheet
Safeguarding your IP: Thermo Fisher Scientific's global commitment to confidentiality
IP property (IP) rights and confidentiality protections play a critical role in biopharmaceutical development and manufacturing. Learn more.

Webinar
Lyophilization excellence: Partnering for sterile fill finish success
This webinar explores the intricacies of lyophilization, including formulation development and cycle optimization, and the growing importance of this critical process for ensuring the stability and efficacy of biologics, vaccines, and advanced therapies.

The quality lever: Shaping success in CDMO partnerships
Compromising on quality can lead to detrimental impacts on both speed and cost, ultimately affecting the successful development and marketing of new therapies.
10 minute read

Blog post
A CDMO Partner for every Gene Therapy Manufacturing Stage
Take a closer look at the experiences of NysnoBio and bluebird bio for insight into what companies need in a CDMO partner for every stage of viral vector manufacturing and development.
20 minute read

Video
Trial Setup and Planning (TSP) solution for clinical trials
Our enhanced Trial Setup and Planning (TSP) system is a cutting-edge solution designed to optimize the efficiency, accuracy, and success of your clinical trial.

Blog post
CDMO 2.0: Three pharma industry trends for 2024 and beyond
Discover three major trends expected in the pharma industry, including turning to flexible manufacturing, embracing digital enablement, and the need for CDMOs deliver transformational value.
15 minute read

Blog post
Benefits of an integrated approach to viral vector manufacturing
Learn why biopharma companies choose to partner with integrated CROs and CDMOs with experience developing and manufacturing viral vectors over doing the work in-house.
15 minute read

Blog post
Understanding the viral vector product journey
Learn why biopharma companies choose to partner with CDMOs to leverage their innovative, integrated, and ready-to-use solutions for viral vector development and manufacturing for gene therapies.
20 minute read

Case study
Accelerating speed to market through solid state & crystallization development: Polymorph case study
Our on-demand webinar, “Accelerating speed to market through solid state & crystallization development,” dives into a polymorph case study in which a more stable polymorph unexpectedly emerged during lab development in preparation for a manufacturing campaign.

Case study
Accelerating speed to market through solid state & crystallization development: Filtration case study
Our on-demand webinar, “Accelerating speed to market through solid state & crystallization development,” dives into a polymorph case study in which a more stable polymorph unexpectedly emerged during lab development in preparation for a manufacturing campaign.
Fact sheet
Translational Services
Through Translational Research Services, our interdisciplinary team of scientific experts offer end-to-end support to advanced therapy developers, to generate relevant materials and help ensure a seamless transition from discovery to clinical phase manufacturing.

Blog post
Exploring four patient-centric trends shaping today’s biopharma landscape
The biopharma industry is adopting a patient-centric approach to drug research, development, and manufacturing. Explore four trends shaping today’s landscape.
15 minute read

eBook
CPHI trend report: The nexus between patient and big pharma
Thermo Fisher Scientific’s new trend report, in collaboration with CPHI, explores how CRO/CDMO collaborations are moving the needle in a brighter, more patient-centric direction.

Webinar
Navigating challenges in HPAPI development and manufacturing
This webinar covers key considerations in developing and manufacturing HPAPIs, including product containment strategies, personnel protection needs, and cross-contamination risks. Learn more...

Webinar
Accelerating speed to market through solid state & crystallization development
Investing in solid state characterization and crystallization development early in the drug development journey can help accelerate speed to market and avoid costly mistakes down the road. Learn more...

Blog post
Sterile injectable therapies: Changing delivery formats revolutionizes lifecycle management
The sterile injectable drug market is evolving at a rapid pace. This segment's significance cannot be understated. A closer examination reveals the intricacies of this evolving landscape, specifically in the realm of delivery formats.
8 minute read

Transforming clinical trial supply chain optimization through digitization
Innovative technologies such as artificial intelligence, automation, and real-time data analytics stand to revolutionize clinical trial supply planning from an efficiency, accuracy, and resiliency standpoint.
15 minute read
Whitepaper
Quantification of AAV vector genome titer and residual host cell DNA using ddPCR
Learn more about the move toward using droplet digital polymerase chain reaction (ddPCR) in the quantification of AAV vector genome titer and residual host cell DNA.

Blog post
Key Insights from CPHI Barcelona 2023
As the largest global event for pharmaceutical supply chain companies, CPHI is a microcosm of the pharmaceutical manufacturing industry. At this year’s event, the rapidly shifting pharma landscape contributed to an undercurrent of urgency. Following are some of the key takeaways.
10 minute read

Webinar
Addressing challenges and key considerations for comparator sourcing in and to China
Comparator sourcing is crucial for clinical trials, but it’s undeniably complex — especially when it takes place overseas. Explore ways to overcome the challenges of comparator sourcing in and to China.
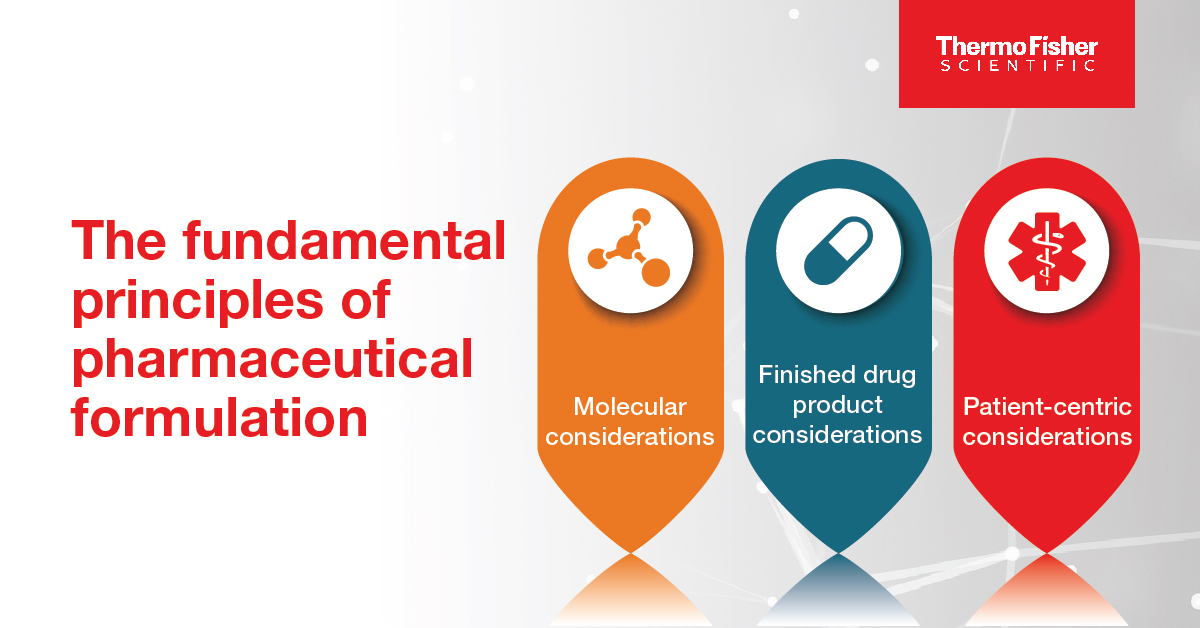
Blog post
Inside pharmaceutical formulation development
Pharmaceutical formulation is a key aspect of drug development and helps to ensure safe, effective, and patient-friendly medications for people worldwide.
15 minute read

Blog post
Unlocking efficiency: Pros and cons of outsourcing your biorepository
When it comes to biorepositories, should biopharma companies insource or outsource their storage needs? This blog breaks down the pros and cons.
8 minute read

Blog post
Building a biorepository: Weighing the benefits and drawbacks
Biorepositories play a crucial role in collecting, preserving, and utilizing biological materials, but does it make sense to build or buy one?
8 minute read
Fact sheet
Viral vector development and manufacturing services
Thermo Fisher Scientific provides over 20 years of unparalleled experience in developing and manufacturing viral vector products.

Webinar
Catalyzing change: The dynamic landscape of CDMO innovation
Explore how the dynamic landscape of the biopharma industry demands constant evolution to meet changing patient needs, satisfy regulatory requirements, and inspire technological advancements.

Fact sheet
Soft lozenge fact sheet
Our new soft lozenge technology is uniquely designed to provide a soft and soothing sensory mouthfeel, allowing for a more comfortable and patient-friendly delivery of active pharmaceutical ingredients.

Webinar
Next-generation analytics in viral vector manufacturing
In this webinar, our analytics experts will provide an in-depth overview of the critical role of analytics in the viral vector production workflow, and talk about pain points like method accuracy, workflow complexity, and throughput limitations. In addition, case studies will demonstrate how innovative methodologies can improve analytical quality and reproducibility.
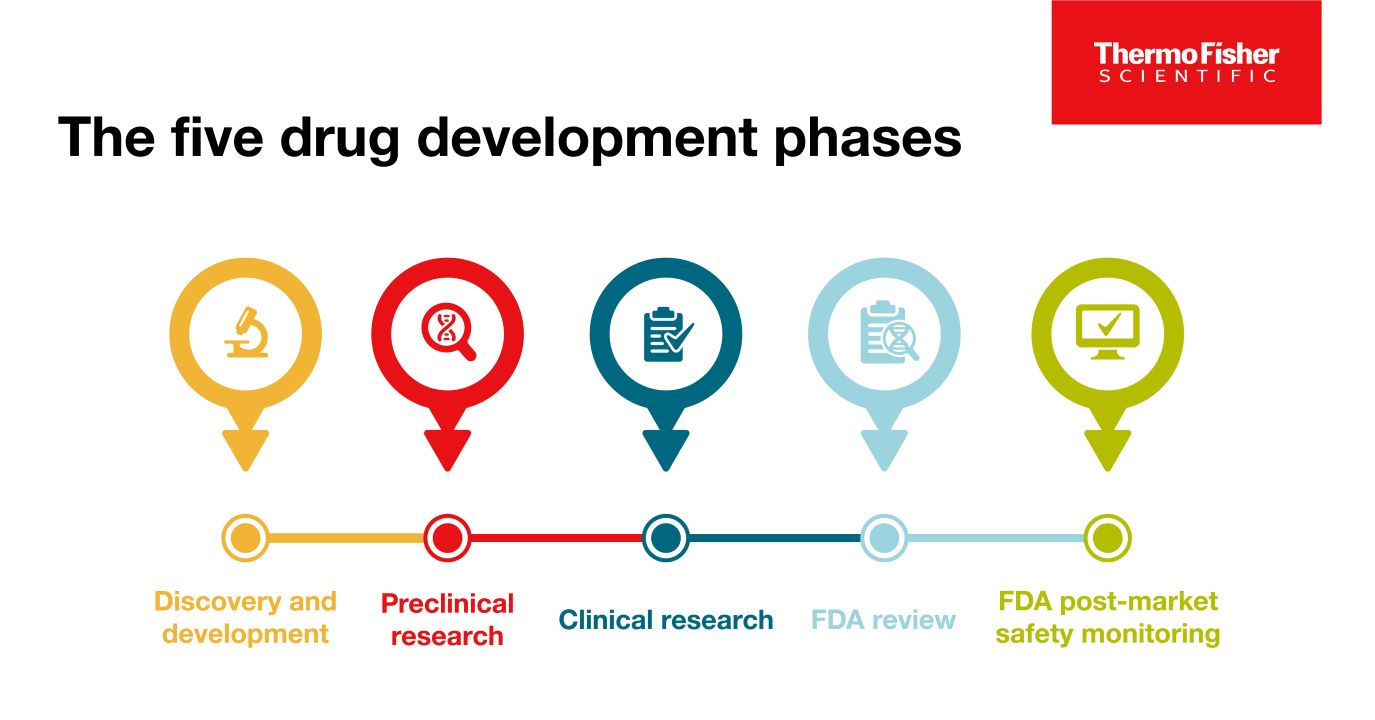
Blog post
The 5 drug development phases
To be deemed a “success,” a new drug must make it through five specific phases: 1) discovery and development, 2) preclinical research, 3) clinical research, 4) FDA review, and 5) safety monitoring.
9 minute read
Blog post
Adherence and Accuracy: Smart packaging advances quality in clinical trials
Learn from Thermo Fisher Scientific’s head of medication adherence and biomarker measurement about data-quality implications of smart packaging and how to integrate them into clinical trials.
15 minute read

Webinar
Launching your First Drug Product: Key Learnings from a Pharmaceutical Start-up
Commercial packaging considerations for the launch of your first drug product

Blog post
In Silico Modeling: Accelerating drug development
In silico modeling, both in early development and across the product lifecycle, can streamline drug development and reduce the risks associated with trial-and-error experimental methods. Realizing the potential of the technology requires careful selection and application of in silico strategies and a deep understanding of how to interpret and derive the most valuable insights from the data.
6 minute read

Blog post
Integrated CDMO for Small Molecule Drug Development
A single CDMO partner that offers integrated API, drug product, and clinical strategy activities can streamline and accelerate small molecule drug development. Learn more.
6 minute read

Webinar
Removing Quality Roadblocks For Cell & Gene Therapies
Industry experts from Lachman, Regenxbio, Thermo Fisher Scientific (PPD) and Etena Therapeutics discuss key consideration of successful CGT manufacturing and quality strategies.

Infographic
Biorepositories: Ten considerations for determining whether to build or buy
Do you need to store your temperature-sensitive critical materials but not sure if you should manage the storage in-house or outsource to a biorepository partner? Explore this interactive infographic to learn about some important factors you may or may not have considered.

Fact sheet
Direct-to-toxicology viral vector services
Toxicology studies are crucial for supporting pre-IND regulatory requirements and evaluating the risk-benefit ratio of drug candidates. Our direct-to-toxicology viral vector services program expedites the route to toxicology materials through our adeno-associated virus (AAV) and lentivirus (LV) production processes, completing them in as little as six months.

Blog post
A collaborative approach to viral vector development and manufacturing
The development and manufacturing of viral vectors from discovery to commercialization is a complicated endeavor with several key considerations. Learn more.
9 minute read
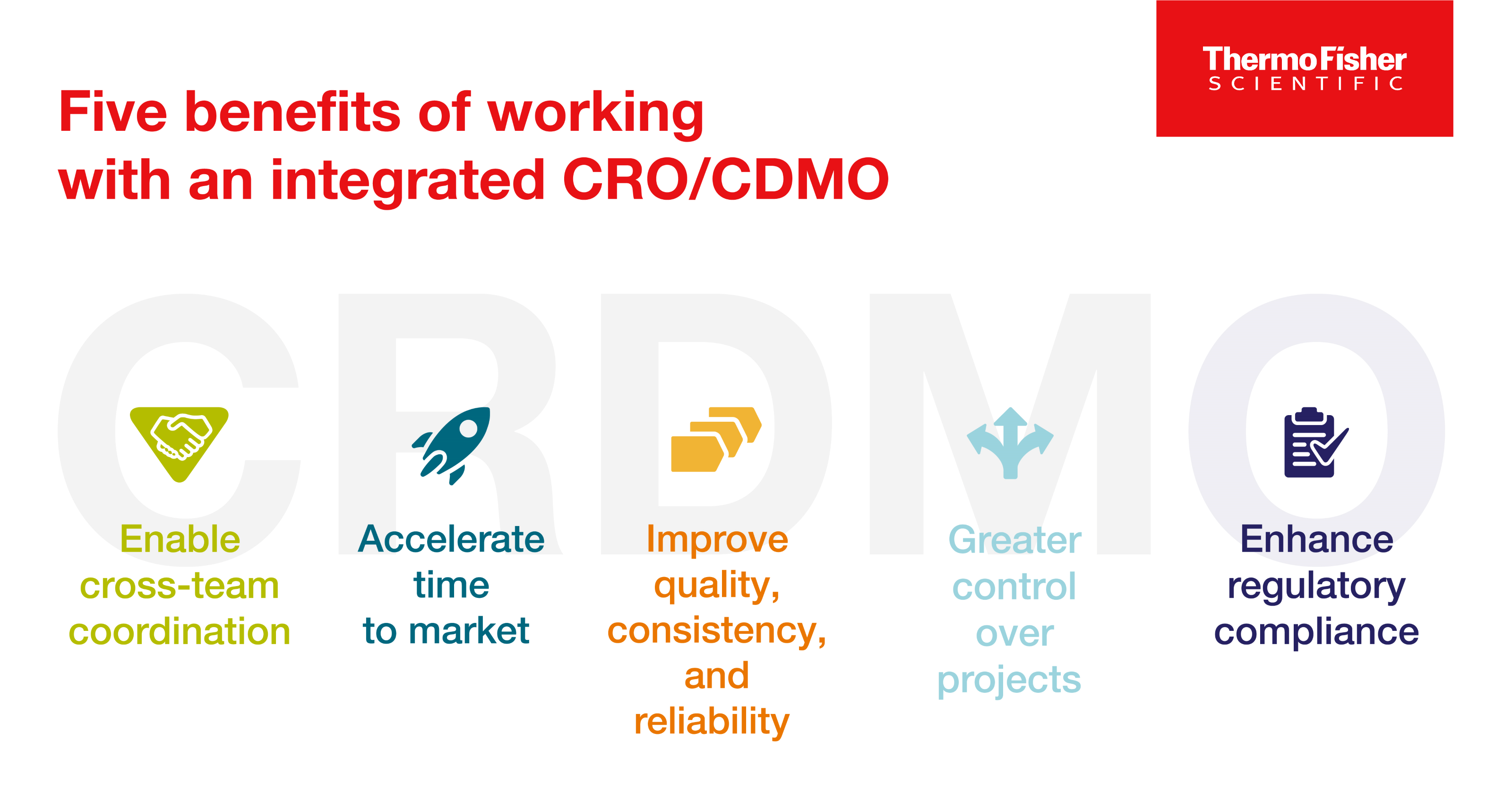
Blog post
Enter the CRDMO: Reshaping drug development through CRO/CDMO integration
CRDMOs, or integrated contract research, development, and manufacturing organizations, are a trend to watch. Discover five benefits of partnering with one.
9 minute read
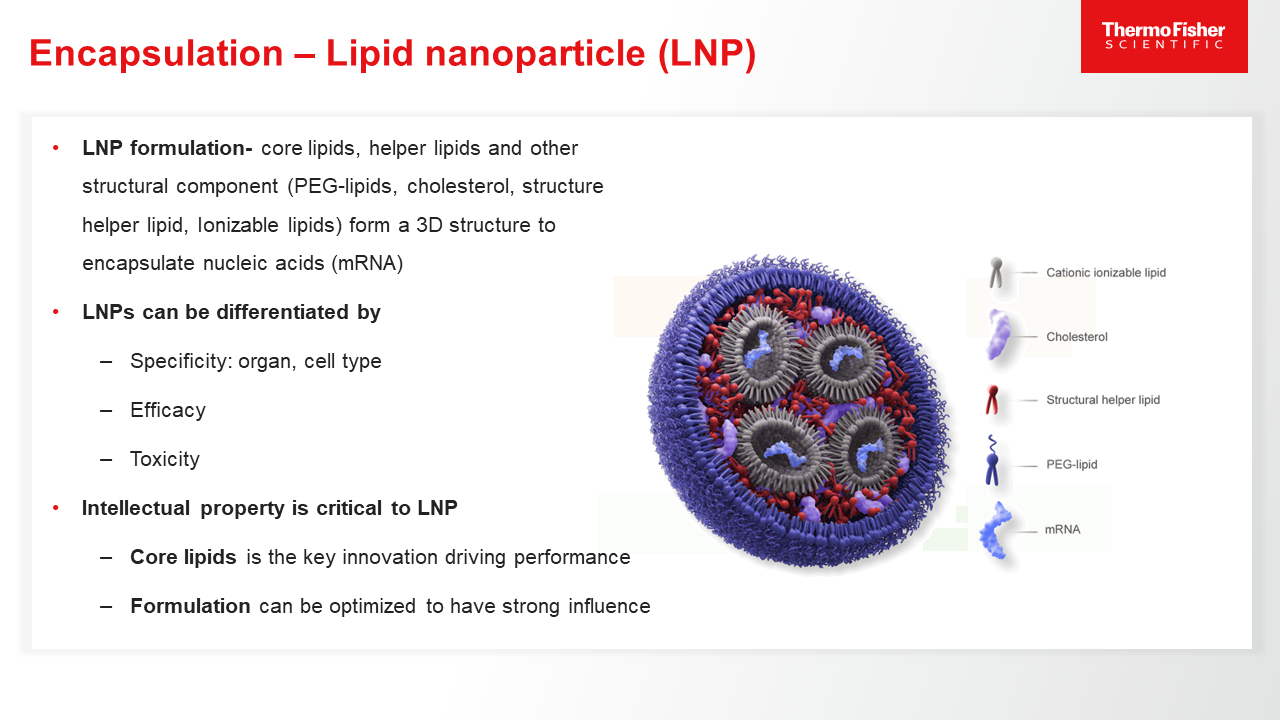
Blog post
Role of mRNA Encapsulation
mRNA must be encapsulated in different carriers/vectors, such as lipid nanoparticles, to ensure its efficient and effective delivery into target cells. Learn more.
10 minute read

Video
Discover our new smart packaging solutions
Thermo Fisher's smart packaging solutions help support improved medication adherence by providing enriched data to better understand the patients and drug behaviors in your clinical trial. Learn more.
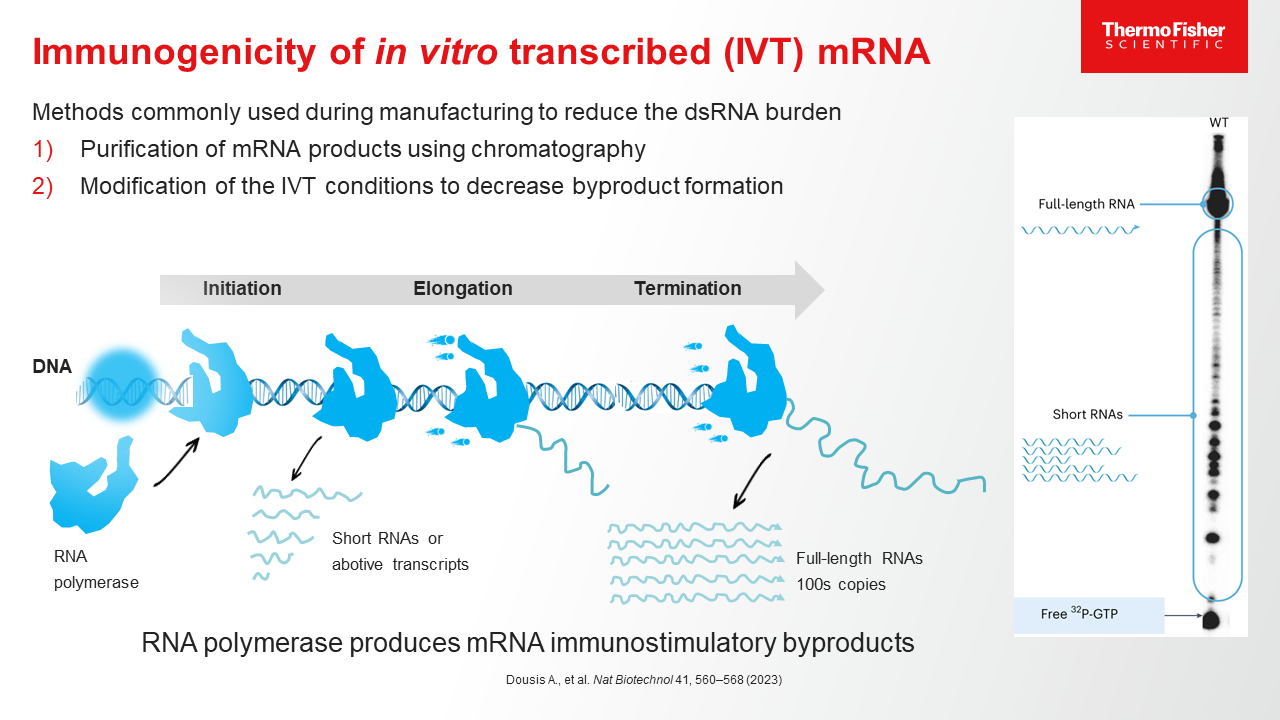
Blog post
mRNA Purification Methods and Process
Learn more about the process of purifying mRNA, and how a CDMO partner with end-to-end mRNA purification experience can help streamline the drug development journey.
10 minute read

Webinar
Navigating a smarter race to market for oral solid-dose products
Watch Thermo Fisher Scientific’s on-demand webinar to learn how to design a development and manufacturing strategy that utilizes quality by design (QbD) principles, digital modeling, and other best practices, opportunities to engage a multidisciplinary approach across formulation development, process engineering, analytical sciences, and regulatory affairs and how an industry-leading, global CDMO partner can benefit programs of any scope or scale — including programs of single-molecule, emerging startups

Webinar
Route Scouting for a cost-effective process development
In this webinar, experts from across Thermo Fisher Scientific’s global network will describe key benefits of route scouting and polymorph screening at early stages of clinical trials to ensure scalability for early clinical supplies to commercial manufacturing.
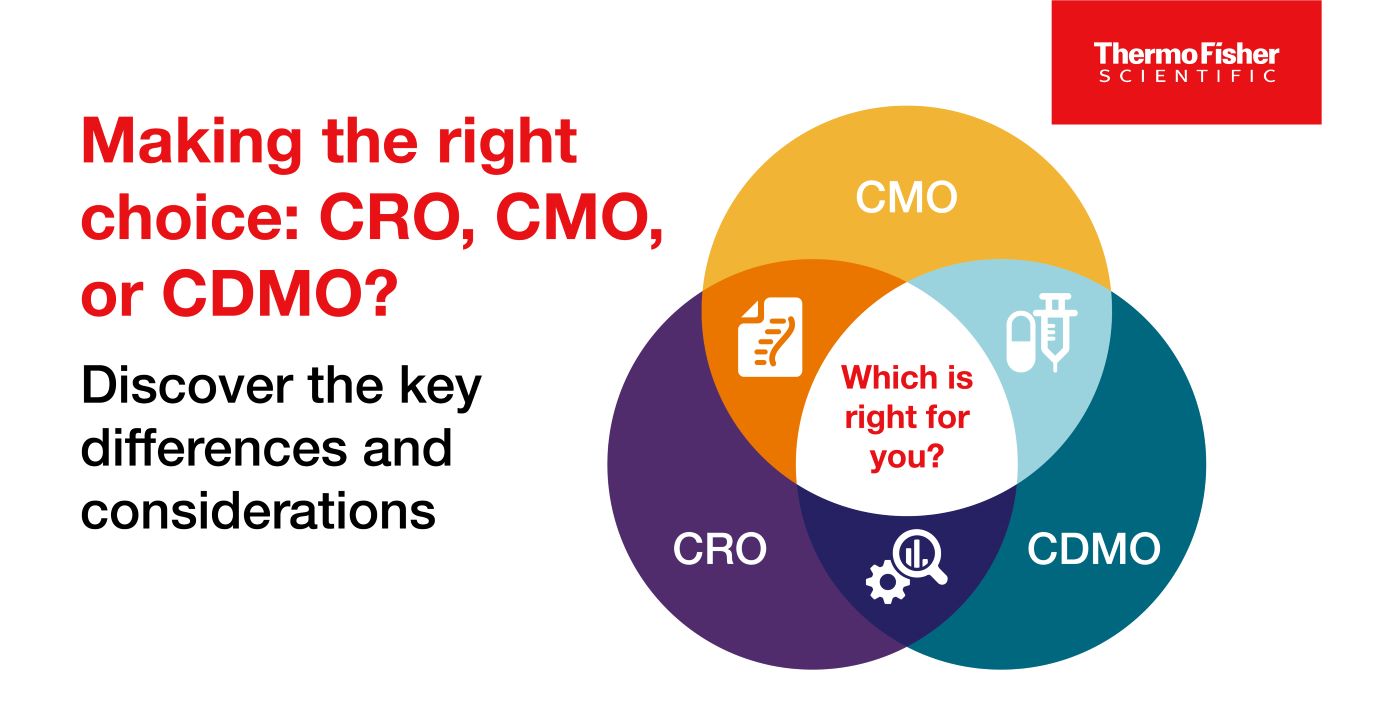
Blog post
CROs vs CMOs, and CDMOs: What’s the difference between the three?
CROs, CMOs, and CDMOs all help biotechnology and pharmaceutical companies with drug development and manufacturing, but what’s the difference between the three?
9 minute read

Webinar
Embracing risk in late-stage biologics drug development
This webinar series explores integrating risk management strategies and mitigating project-specific PPQ risks, as well as optimizing analytical methods for a seamless product launch.

Blog post
Product and partnership quality in viral vector manufacturing: Your gene therapy depends on it
Viral vectors are inherently complex to produce at scale, requiring a laser focus on quality to ensure the efficiency, safety, targeted delivery, and scalability of the gene therapy.
7 minute read

Infographic
Ensuring quality consistency: Thermo Fisher Scientific’s global commitment to quality
Learn more about our quality framework initiatives, tools, and methodologies for maintaining quality standards across the entire drug development process.

Fact sheet
Patheon Quick to Clinic™ Viral vector services
Using Thermo Fisher Scientific's end-to-end manufacturing service, we deliver high-performance, scalable, AAV and lentiviral vectors to clinics. As a result of this service, you can deliver therapy to clinics while de-risking timelines and global regulatory filings without additional or surprising out-of-pocket costs.

Blog post
What is a CDMO and what to look for in a partner
Learn how CDMOs (contract development and manufacturing organizations) work with pharma companies, and the top considerations companies have when choosing a CDMO partner.
9 minute read

Whitepaper
Transforming CDMO partnerships through a holistic understanding of quality
Get an in-depth look into key indicators of CDMO quality, with tools and best practices to drive continuous improvement, strengthen collaboration, and ultimately cultivate trust.

Fact sheet
Bioservices lab overview
Providing timely and accurate analysis of critical samples in our BSL-2 and CL3 laboratories strategically located within our biorepositories to ensure sample integrity. Learn more.
Article
Interactive article: Addressing challenges in mRNA drug development and manufacturing
Learn about five specific challenges in mRNA drug development and manufacturing, and the avenues to overcome them.

Blog post
Viral vector commercialization – Part 3: Specialized regulatory support
Find detailed regulatory considerations when preparing viral vectors for commercialization and best practices to address them.
7 minute read

Webinar
Benefits of an integrated approach to gene therapy development and manufacturing
Thermo Fisher Scientific's expert will take you through the development and commercialization of viral vectors for gene therapy, so you can navigate these hurdles and deliver the project in a timely, cost-effective manner. With integrated gene therapy development and manufacturing, you can benefit more than a customized solution. It improves coordination, streamlines decision-making, and uses resources more efficiently.

Webinar
QP Release in the EU clinical trial regulation: Lessons learned one year on
Watch this webinar to hear from a Qualified Person (Q)P as they answer pre-submitted questions about the EU Clinical Trials Regulation, how to ensure the timely supply of Investigational Medicinal Products (IMPs) and more.

Webinar
Optimizing the cell therapy patient journey through integrated CRO CDMO partnership
Watch this on-demand webinar for insights on how working with a single integrated CRO/CDMO partner can help ease industry challenges and provide an accelerated path from development to manufacturing, as well as the benefits that come from unified teams and infrastructure.

Webinar
Addressing industry challenges in mRNA product commercialization
The rapid development and approval of mRNA-based vaccines during the COVID-19 pandemic has spurred a renewed interest in mRNA technology, with scientists exploring new application areas such as oncology, HIV, rare diseases, and even personalized medicine. While the speed of production and flexibility of mRNA are appealing, several industry challenges must still be addressed to realize the full potential and further expand its use. Learn more.
Webinar
Solving for solubility: a scale up strategy for spray dried dispersions
Thermo Fisher Scientific’s experts will describe key considerations for progressing spray dried dispersions from early formulation-screening through process development and scale-up for early clinical supplies to commercial manufacturing.
Fact sheet
Critical Biological Material Management
We ensure the integrity of temperature-sensitive critical material – from collection and storage through delivery to patient – with customized, end-to-end cold chain supply management solutions.

Fact sheet
Process Research Group: Route Scouting
Route scouting is an essential step in the chemical development process for drug substances (API). Learn about our Chemical Process Research Group, dedicated to process research on new routes.

Blog post
Viral vector commercialization – Part 2: Best practices in process validation lifecycle
Learn more about the robust viral vector process validation cycle, which includes various assessments and studies to ensure the safety, efficacy, and quality of viral vectors.
11 minute read

Fact sheet
Quick to Clinic™ for Small Molecule Oral Solid Dose
Our Quick to Clinic™ for small molecule oral solid dose offers in-silico modeling, API and solid-state chemistry, and analytics and formulation development capabilities. Learn more.
Infographic
Preparing biologics for commercialization: Understanding Strategies to Reduce Risk and Optimize Outcomes in Drug Development
Within the drug development process, there are several steps that occur between the laboratory and final manufacture of the drug product. Different players step in during each point, so keeping a program with many moving parts on track requires planning and time-tested execution approaches.

Fact sheet
A comprehensive approach to improving solubility and bioavailability: Spray drying
Spray drying provides a strategic solution to address bioavailability or crystallization challenges that are common across many drug substances and drug products. Thermo Fisher provides spray drying strategies that can be leveraged from early development to the commercial scale.

Infographic
Cold chain services for clinical trial success
This interactive infographic highlights Patheon’s capabilities for maintaining cold chain integrity across the supply chain. Utilizing our global network and expertise, our end-to-end cold chain management services can support your advanced clinical trial needs.

Case study
Thermo Fisher Scientific supports rapid global COVID-19 manufacture
Learn how a pharmaceutical company developed a successful vaccine and delivered millions of doses, on time to the waiting world, in a historical step forward in the fight against COVID-19.

Video
Complete capabilities for cold and ultracold clinical supply chain management
Learn more about our capabilities for cold and ultracold clinical supply chain management.

Case study
ATMP Fast Track: From clinical to commercial
A leading allogeneic T-cell immunotherapy company needed expertise and help ushering an innovative therapy from academia through to commercialization.
Download the case study
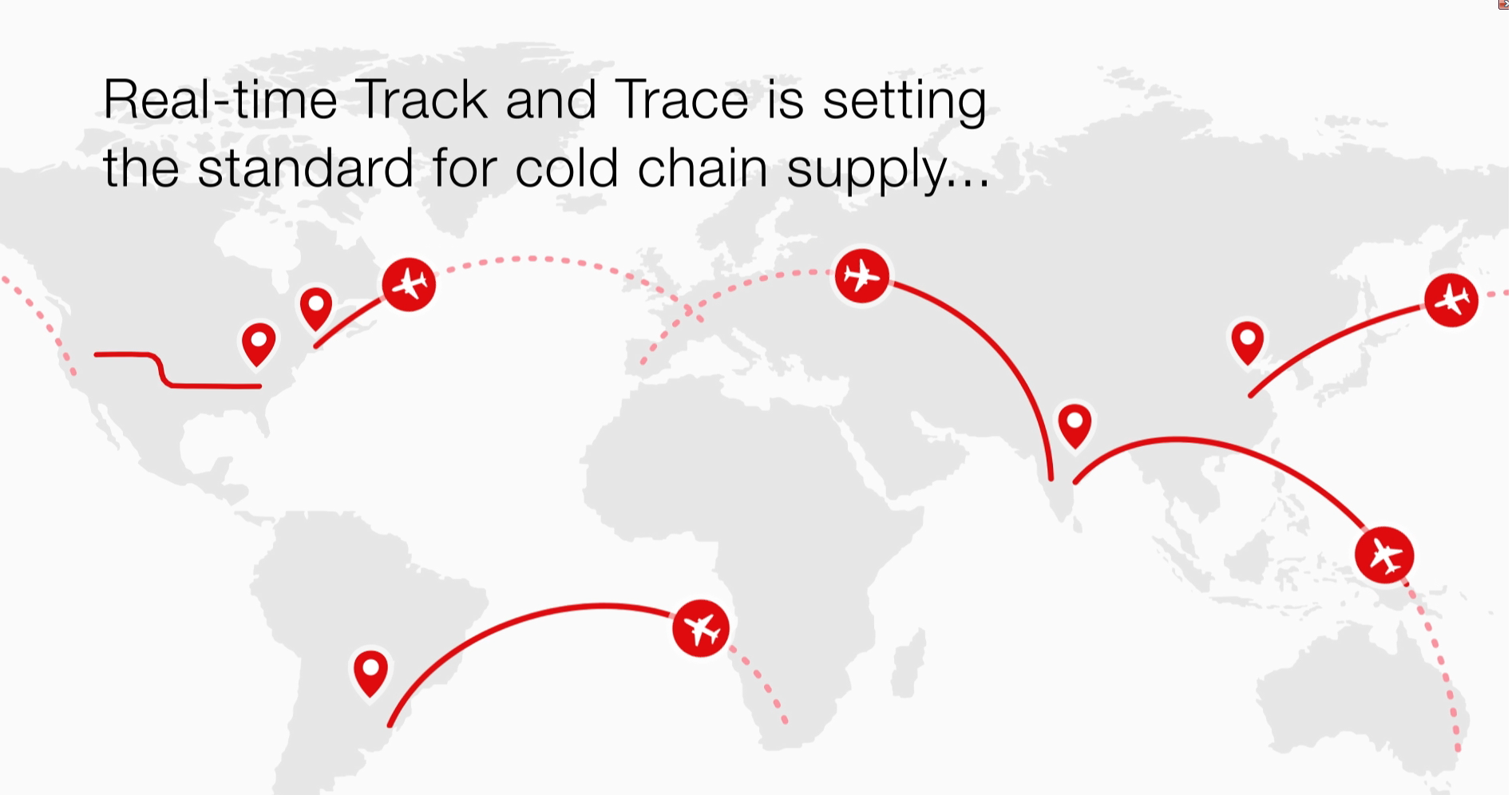
Video
Real time Track and Trace
Learn more about Thermo Fisher Scientific’s Real time Track and Trace solution which provides internal and external clinical trial stakeholders with on-demand shipment updates for all in-transit materials for a clinical trial.

Blog post
Viral vector commercialization – Part 1: Tech transfer process for commercial viral vector manufacturing
Learn how tech transfers can help develop and manufacture viral vectors at scale, accelerate vaccine and gene therapy commercialization, and provide expertise.
9 minute read

Infographic
Considerations for clinical trial success.
Explore this infographic to arm you with important factors to consider when embarking on clinical trials.
Webinar
Avoiding the crushing cost of poor quality in biopharma through digital innovation and strategic collaboration
In this webinar, we will discuss customer-centric digital transformation that are improving overall process and product quality and performance and transforming CDMO relationships.

Video
mRNA Fast Facts
This video provides a summary of Thermo Fisher Scientific's end-to-end mRNA development and manufacturing capabilities.

Blog post
Patheon Translational Services Advance Cell and Gene Therapies from Research to Clinical Trials
Learn how our translational research services, housed in our San Diego facility, can take cell and gene therapies from preclinical to clinical.
6 minute read

Whitepaper
Evolving solutions to optimize clinical trial decentralization
This whitepaper highlights Thermo Fisher's solutions to drive high adherence in decentralized trials.

Infographic
Regulatory pathways for CGT and ATMP products
CGT is one of the world's fastest-growing therapeutic areas today. Instead of treating patients for the rest of their lives, these therapies offer them hope of a cure.
In this infographic, we will review three tips for achieving regulatory success.

Infographic
Top 10 traits to look for in a CDMO partner for cell, gene, or advanced therapy medicinal products
This guide can help ease the CDMO selection process by identifying key areas of conflict and uncertainty in the partnership and production process. By proactively asking questions and addressing concerns with CDMO candidates, pharmaceutical companies can minimize conflicts down the line and ensure they have selected a partner that maximizes their chance of success.

Blog post
Top tips for providing the right amount of detail in first-in-human common technical documents
In the early-development stage, little may be known about a drug’s characteristics. What’s more, drug processes and formulations frequently evolve as more information emerges following testing and trials. Learn more.
5 minute read

Infographic
Cell therapy manufacturing workflow
View our new infographic for an overview of a genetically modified cell therapy workflow and the key considerations at each step, ranging from plasmid production through cold chain logistics.

eBook
Cell and gene therapies in the US vs. the EU: Top five areas of differentiation
In this eBook we share the five key differences in the drug development and review process for companies hoping to gain market access through US Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval—as well as tips for navigating these differences.

Infographic
A practical guide to writing robust chemistry, manufacturing, and controls dossier modules to support first-in-human trials
Do you need to get your first-in-human (FIH) paperwork in order? This informative checklist can help you draft phase-appropriate chemistry, manufacturing, and controls (CMC) modules for the common technical document (CTD).

Blog post
Enabling a digital culture through integrated business processes
In contrast to a physical work environment, where stability and experience are key, a digital business environment focuses on innovation and connectivity. Learn more.
4 minute read

Blog post
Delivering on the promise of cell and gene therapies: A patient-centric approach
In this session, you will gain insights into the complexities of the CGT ecosystem and the challenges that must be overcome to successfully move CGT therapeutics from the laboratory to the patient.
8 minute read

Infographic
Looking both ways for your small biopharma clients
Learn which CDMO partner can lead your biopharma clients down the ideal path and keep client’s projects on time, and on track for regulatory and market success.

Webinar
Enabling a digital culture through integrated business processes
In this webinar, we share our perspective for achieving digital transformation and digital culture enablement, focusing on innovative solutions, opportunities for attaining new levels of operational excellence through proven technologies to drive digital transformation and the Pharma 4.0 journey, and key considerations for transforming the traditional supply chain model into a digitally integrated value chain network

Blog post
Gaining a deeper understanding of your API
As active pharmaceutical ingredients (APIs) become increasingly complex, they pose potential formulation problems that can extend timelines and explode budgets. Read this blog to learn more.
5 minute read

Case study
Midsize pharma company stabilizes API - Accelerates time to clinic from three months to three weeks
A midsize pharma company wanted to progress its therapy from early development to late-phase clinical trial stage, but needed a CDMO partner to provide support in formulation analysis so that it could stabilize its product and successfully reach its clinical studies milestones.
Poster
Accelerating path to clinic through an end-to-end solution focused on supply chain, regulatory, and a robust manufacturing process for viral vectors
Enhancing the path to clinic with an end-to-end solution that addresses supply chain, regulatory, and robust manufacturing processes for viral vectors.
Learn more about a true end-to-end offering for AAV and LV vector manufacturing.

Webinar
Navigating the EU regulatory landscape: Accelerating drug development, treatment innovation and market access
During this three-part webinar series, we cover key areas sponsors must be mindful of during drug development in preparation for filing successful regulators submissions.
Blog post
EU and US regulations: What’s coming for cell and gene therapies?
Cell and gene therapy (CGT) developers today face an added challenge in their quest to bring a product through clinical trials and to the market. Read this blog to learn more.
7 minute read
Infographic
CDMO checklist to launch your molecule globally
Preparing to take your drug into the global market? You’ll need to make sure your CDMO has what it takes to successfully navigate the global regulatory space with speed, security, and supply safeguards. Use this quick list as a reference when evaluating your options.

Webinar
Future Therapies Track Keynote: Delivering on the promise of cell and gene therapies
Gain insights into the complexities of the CGT ecosystem, and the challenges that must be overcome to successfully move these therapeutics from the laboratory to the patient. Learn about best practices, tools, and integrated solutions for streamlining CGT development and key areas of focus for improving the journey of patients enrolled in CGT clinical trials and beyond.

Video
Autologous and allogenic therapies - today and tomorrow
In this roundtable discussion, clinical and commercial experts address the complex dynamics associated with allogeneic vs. autologous cell therapies and offer insight into the current and future state of the industry. Specific topics include best practices for manufacturing and logistics, regulatory landscape and CMC requirements, and the role of standardization.

Webinar
Addressing cell and gene therapy supply chain challenges through global, integrated solutions
The following webinar features insights from industry experts to help you mitigate supply chain risk and accelerate access to new cell and gene therapies.

Brochure
Real Time Track and Trace
Real Time Track and Trace supports enhanced shipment visibility by enabling
tracking of all shipments in one place, viewing cold chain shipments using real-time temperature and location tracking, real-time tracking of delays and excursions, and advanced monitoring for critical cold chain and high value product shipments. Real-Time track and trace keeps the client informed every step of the way

Whitepaper
Comparator sourcing: Leveraging the Bolar exemption to accelerate market access for generic and biosimilar products
Thermo Fisher's Comparator team has the required expertise to leverage the Bolar exemption, providing clients early access to generic / biosimilar drugs.
Strategies to accelerate drug development through harmonization of early and late stage processes
This technical article presents a harmonized and streamlined approach established at Thermo Fisher Scientific for manufacturing AAV and LV vectors for discovery research using technologies and processes mirroring current GMP platforms.

Webinar
Preparing your biologic for commercialization: strategies to reduce risk and optimize outcomes
Leverage the experience of a top CDMO for best practices including how to remain flexible in the face of uncertain market demand, identifying the best practices to a reliable execution, and optimizing the latest digital tools to reduce risk to your timelines

Case study
Thermo Fisher partners with orphan drug developer to resolve dissolution challenges
Learn how Thermo Fisher Scientific’s pharma services team used sophisticated imaging techniques and stability studies to help a pharmaceutical company resolve instability issues, threatening patient safety and successful use of the drug in a Phase III clinical trial.

Webinar
Patient-centric sterile injectables: Improving patient experience through formulation and delivery
Join us for an expert panel of biologics experts as we discuss patient-related characteristics that should inform drug product design decisions, product-related characteristics that can be modified to address the needs of the target patient populations and innovative technologies that can be used to address patient needs

eBook
Decentralized clinical trials in the EU - Strategies and digital solutions for navigating the regulatory landscape
Download this eBook to learn how to navigate the complexity of the regulatory landscape in the EU with the right guidance and resources.

Webinar
Material matters: Material science, process simulation and modeling for oral solid dosage forms
In the development of oral solid dosage forms, predictive modeling and simulation tools of the manufacturing processes can enhance process understanding, save on API supply, improve product quality, and reduce or eliminate many of the bottlenecks associated with empirical methods.

Webinar
Accelerating your biologic’s development: From lab, to clinic, to market
Learn how Thermo Fisher Scientific is supporting new and emerging biotechs to get to clinic and to market, faster. No longer viewed as transactional service providers, CDMOs are an integral part of the pharmaceutical supply chain, and collaboration is foundational to the drug sponsors’ success.

Whitepaper
Targeting cancer with therapeutic antibodies: Solutions for every phase of mAb development
This whitepaper identifies the key challenges faced at each stage of mAb development and provides guidance for streamlining pipeline progress of therapeutic mAb candidates.

Whitepaper
Switching delivery formats: A lifecycle management strategy for sterile injectables
Switching delivery formats can be an effective strategy to enhance they lifecycle of your drug. This whitepaper provides insight into the benefits and challenges of transferring a therapeutic compound from one aseptic injectable format to another.

Webinar
Smart packaging in clinical trials: What clinical supply teams need to know
This webinar is a mix of a short didactic presentation and a panel discussion with clinical supply innovators from across the industry, sharing their experience and lessons learned as they work to normalize the use of adherence measurement tools and smart medicine packages.

Fact sheet
Customs warehouse solution
Learn how our customs warehouse solution can positively impact your overall material import and export costs.

Infographic
The 5 benefits of a customs warehouse solution
See how our customs warehouse solution can simplify your import/export strategy with our interactive infographic.

Infographic
mRNA manufacturing workflow
Like many therapeutic manufacturing workflows, every step in the mRNA process builds upon the prior step. This infographic explores each intertwined step in the mRNA manufacturing process and outlines how Thermo Fisher Scientific’s flexible approach can get your mRNA product to clinic and market faster.

Fact sheet
Commercial autoinjector capabilities
Learn about our commercial autoinjector packaging, labeling and kitting capabilities.

Webinar
Flexible regulatory pathways and key CMC considerations to commercialize cell and gene therapy products
This webinar will provide key advice for determining and navigating the regulatory pathway for any cell and gene therapy.

Webinar
Risk assessment strategies in oral solid dosage development and manufacturing
When you reach each stage in your drug development journey, there are numerous items to look out for as you look to scale up your product.

Fact sheet
Small molecule API solutions
Whether you require standalone API services or fully integrated end to end drug development, we’ll act as extension of your team. Combining the power of deep scientific expertise, industry leading technology, and the backbone of a global network, we’re dedicated to get you closer to your goal.

Whitepaper
Expediting early-phase development of small molecules: An integrated approach
Small molecule drug development has changed substantially in recent years. With the heightened focus on molecularly targeted therapies, small molecule active pharmaceutical ingredients (APIs) and drug products are more complex and potent than ever, requiring increasingly specialized
manufacturing processes and drug delivery solutions. At the same time, the competitive demand for rapid entry into clinical development—combined with accelerated review pathways—translate into compressed manufacturing and delivery timelines.

Infographic
4 time-saving solutions to common early development challenges
In the race to market, you need fast, cost-effective, and scientifically proven solutions to seamlessly guide your molecule through early-phase development. See how we can offer you time saving solutions for your early development.

eBook
Protecting tomorrow: Supporting pharmaceutical and biotech industries to build a sustainable future
Learn how Thermo Fisher is meeting its environmental sustainability goals, and how we work in partnership with the pharma and biotech communities on shared environmental sustainability goals.

Whitepaper
Safety first: Controlling occupational exposure in oncology drug development
This whitepaper provides a roadmap for assessing toxicological and potency risks of small molecule oncology compounds, focusing specifically on the following considerations: Criteria for evaluating highly potent small molecules, toxicity banding systems, potency downgrading and safety and handling strategies.

Whitepaper
The Economic Advantage of Single-Source Drug Development and Manufacturing
Tufts Center for the Study of Drug Development (CSDD) recently completed research, titled “Assessing the Economics of Single-Source vs. Multi-Vendor Manufacturing” that compared cycle times and development economics between...

Spray Drying Processability of Methacrylic Acid Copolymers in Amorphous Dispersions: A QbD Approach
Download the AAPS Best Abstract Award winner from our Bend site that showcases our capabilities, expertise and experience in spray drying.

Case study
Delivering a large-scale product at a rapid pace
When a pharmaceutical client needed to introduce, manufacture, and validate five stages of challenging chemistry to support a Phase III clinical development program, the Thermo Fisher Scientific pharma services team had to get to work.
eBook
To err is human, to correctly formulate is divine: Risky assumptions for addressing limited bioavailability
Finding a safe and effective compound to bring to market is challenging and costly enough – avoid risking additional expense, be it time or money, by not making these assumptions about the importance of a well planned and executed formulation development strategy.

Fact sheet
Total transportation management
Total Transportation Management manages the complex supply chain processes required to move all types of life science shipments both domestically and internationally.

Fact sheet
Global packaging solutions
Leveraging over 30 years of global clinical packaging and labeling expertise, Thermo Fisher Scientific offers flexible clinical supply services that can help streamline and add speed to your packaging and labeling workflow.

Fact sheet
Solutions for high potency
Executing an efficient and optimized high potency strategy early builds a strong foundation when it comes time to scale up manufacturing.

Infographic
Advances in viral vector manufacturing
No one viral expression system is right for every situation. There will always be tradeoffs and choices to make. This infographic is intended to provide thinking points to help you consider options before deciding which path to take.

Infographic
The unrealized value of a combination drugs strategy
Fixed-dose combination drugs are increasing in popularity due to their clinical benefits over monotherapy alternatives, but there are also several business advantages drug sponsors can derive from utilizing a combination drug strategy. Download the infographic to learn more.

Presentation
Preparing viral vector productions for commercialization
Gene therapy vectors are rapidly approaching the commercial space so commercial readiness is critical for success. Watch our webinar to learn about our capabilities and approaches for preparing viral vectors for commercialization.

Presentation
Quick to Clinic™ viral vector platform
Learn how to leverage scalable, standardized platform process for suspension-based AAV and LV vector manufacture to accelerate time to clinic with this webinar

Blog post
Choosing a CDMO for mRNA success: Five CDMO characteristics needed
The promise of mRNA technologies has been clearly demonstrated during the COVID-19 pandemic, with vaccines reaching the market in record time. The vital role...
7 minute read

Blog post
Trends in mRNA therapeutics: Pandemic learnings for a pathway to success
The rapid advancement of the Pfizer-BioNTech and Moderna messenger RNA–based COVID-19 vaccines from lab to clinic—with development taking less than one year—has validated the...
5 minute read

eBook
Biosimilars: Top 4 differences when managing clinical trial supplies
This eBook outlines the top 4 differences in managing clinical supplies for biosimilar trials. These challenges make biosimilar studies anything but routine for clinical supply teams.

eBook
What are passive shippers and how do they work?
These eBooks outline industry trends and reviews how Fisher Clinical Services solutions are meeting the challenges of the cold chain distribution of clinical
trial supplies all over the world.

Fact sheet
Total transportation management Global supply chain oversight
A fully integrated transportation management service across our clients’ supply chain, from discovery through commercial distribution, with unmatched quality & service.

Infographic
Five tips for managing your clinical ancillary materials
Learn more about the advantages of advance planning when it comes to Clinical Ancillary Supplies.
Article
QP release in the EU in 2022 and beyond: Your questions answered
In a recent webinar titled “QP release in the EU in 2022 and beyond: Your questions answered,” two clinical QP experts from Thermo Fisher
Scientific discussed the expectations and responsibilities of the UK and EU QPs in ensuring supply of clinical and commercial drug products between the UK and the EU. Their observations and responses to key questions are shared in this article.

Fact Sheet
Solutions for formulation challenges
Solutions for formulation challenges Formulation challenges for enhancing the bioavailability of poorly soluble APls can be solved by spray drying. We have extensive capabilities and expertise in process development,...

Fact sheet
Global regulatory services backed by industry experts
Navigating a complex regulatory environment is vital to the success of your product’s lifecycle. Thermo Fisher Scientific provides a range of flexible regulatory solutions that can help easily address your molecule’s unique needs and challenges, while being backed by a global network and seasoned regulatory experts.

Webinar
Delivered with options & optimism
Behind every successful project is a team of problem solvers, especially when faced with the challenges of a global pandemic.

Webinar
The future of decentralized clinical trials in an evolving EU regulatory landscape
The COVID-19 pandemic has demonstrated the feasibility of adapting clinical trials from site-based endeavors to fully decentralized or hybrid models and has increased global acceptance of decentralized trials as a safe and effective option for accelerating clinical research.

Webinar
Going global: The impact of US foreign trade zones on drug product manufacturing costs and timelines
Learn more about the impact of US foreign trade zones on drug product manufacturing costs and timelines.

Webinar
entering-first-in-human-clinical-trials-a-five-point-strategy-for-building-a-robust-cmc-dossier
This webinar discusses the five strategies to build a robust CMC package, help streamline the path to FIH trials for biologics and establish the quality foundation needed to support all of the development phases toward commercialization

Whitepaper
The changing landscape of oncology drug development: Bringing novel lifesaving therapies to patients
Oncology is the fastest-growing, most active sector of drug development. Matching drug products to clinical and commercial needs requires scientific and technological innovation.

Whitepaper
Technology transfers: Best practices to mitigate risks and to ensure reliable product technology transfers
This whitepaper offers practical insights into the critical technology transfer process in the pharma industry, outlining common challenges and offering case-based solutions and best practices.

Video
Made with N-of-1 & all-for-one
What seemed impossible became achievable when Thermo Fisher Scientific manufactured and released a one-of-a-kind treatment in a record timeline of 30 days.

Video
Solved with intelligence & imagination
When a client faced a complicated packaging challenge, Thermo Fisher Scientific’s Lead Account Manager went to the drawing board, literally.

Video
United with capabilities & compassion
Is it possible to bring your molecule to medicine without a brick and mortar site and no testing labs or distribution capabilities?

Video
United with process & partnership
An experienced CDMO partner with an extensive network makes the difference when it comes to scale-up, tech transfer capabilities, and validation.

Video
Delivered with options & optimism
Behind every successful project is a team of problem solvers, especially when faced with the challenges of a global pandemic.

Webinar
Key considerations when selecting a CDMO partner for cell therapy manufacturing
The cell and gene therapy market is experiencing accelerated market approvals, record breaking investments, robust therapeutic pipelines, and positive clinical outcomes – all driving the need for speed, regulatory know-how, and innovation in manufacturing technologies.

Webinar
Form and fit: Mobilizing integrated resources to transform complex small molecules into high-performing drugs
As clients seek to gain maximum benefit from each clinical stage, the advantages offered by contract partners with integrated services are making these higher returns tangible.

Webinar
Breakthrough Therapy Designation: Evaluation of trends and impact on CMC Strategies
Breakthrough Therapy Designation (BTD) was introduced by the US FDA to help shorten the development and review time of novel drugs intended to treat serious or life-threatening diseases.

Webinar
Stabilizing your supply chain in times of global volatility
A stable supply chain is critical to making sure your drug product is manufactured and delivered to patients on time.
Poster
Hot Melt Extrusion Process Optimization and Formulation Development of Amorphous Solid Dispersions for a Poorly Soluble Calcium Channel Blocker
Poster
Evaluate Impact of Spray Drying Process Parameters and Equipment Type on the Granule Characteristic of the Immediate Release Tablets of a BCS Class-IV Drug
Poster
Developing a High API Load Immediate Release Tablet by Switching from a Wet to a Dry Granulation and DoE Formulation
Poster
Poster
Evaluation of a Quality by Design (QbD) Approach for Process Development and Scale-Up of a Novel ALS Drug Product
Poster
Determine the Dosing Disk and Encapsulation Speed for Encapsulation of a Drug Product Using Automated Encapsulator
Poster
Enhanced Solubilization of an Analgesic/Antipyretic Drug With The Use of Polymeric Excipients
Poster
Poster
Poster
Comparative PK Performance of Solution and Suspension Based Formulations For a BCS Class II Molecule in a Soft Gelatin Based Dosage Form
Poster
Development of a High Shear Wet Granulation Process Through the Use of Design of Experiments
Poster

Brochure
Solid and Sterile Dose Form Overview
Oral Solids. Access a remarkable range of conventional and specialized oral solid dosage form capabilities and scale. Further expand your options with innovative combinations of these forms and a...
Top strategic tips for filing a successful IND with speed and efficiency
Top strategic tips for filing a successful IND with speed and efficiency Planning for your IND submission takes careful consideration and the right people on your team.

Top strategic tips for filing a successful IND/IMPD with speed and efficiency
Planning for your IND/IMPD submission takes careful consideration and the right people on your team. With proper planning, you can get your filing right the first time and avoid rework later as you expand scope and markets. View the infographic for 8 strategic tips that can take you from confused to confident in your regulatory journey.

Real-time Track and Trace platform for clinical trial shipments
Real-time Track and Trace platform for clinical trial shipments Real-time Track and Trace platform for clinical trial shipments Thermo Fisher Scientific’s Track and Trace solutions provide internal and external...

Whitepaper
Phase Appropriate Formulation and Process Design
Pharmaceutical companies walk a tightrope in early drug development. They have to balance speed, quality, scientific risk, and API cost. Compromising on any one of these four crucial elements...

mRNA vaccine development: Key insights for planning, workflow, and supply chain success
In this report, we will review key insights derived from the development of mRNA-based COVID-19 vaccines and how they can be applied to accelerate progress in the manufacture of mRNA vaccines and...

European regulatory landscape: Demystifying new Qualified Person (QP) requirements for supplying medicine in the EU and UK
To ensure full compliance and timely and efficient batch release processes, drug sponsors pursuing clinical trials and commercialization in the EU and UK require a deep understanding of European...

Article
Q&A: BIO EXPERTS: Post-discovery priorities: Streamlining your molecule’s route to IND
Time to market is paramount for many companies starting to consider post-discovery development and strategic plans for bringing a molecule into clinical trials and beyond. Strategies and tactics for shortening timelines often involve trade-offs related to risk and future needs, making early-phase decision-making a balancing act for even the most promising molecules. Read what our experts how to say about the critical considerations that impact timing and commercialization.

Article
Understanding the roles of solid-state characterization and crystallization within the product lifecycle
Understanding the roles of solid-state characterization and crystallization within the product lifecycle The physical properties of a solid active pharmaceutical ingredient (API) dictate the crystallization process used for isolation,...

Article
Insider insights – Q&A with Tom Holmes of Amylyx Pharmaceuticals
When two young innovators, Justin Klee and Joshua Cohen, Co-Founders and Co-CEOs of Amylyx Pharmaceuticals, discovered a potentially life-changing molecule in their dorm room at Brown University, they embarked on a journey to clinical trials to help treat people living with ALS. Amylyx’ Global Head of Supply Chain, Tom Holmes, shares more about their experience teaming up with Thermo Fisher Scientific to manufacture their product.

Fact sheet
Smart Packaging – Gain Confidence in Clinical Trial Outcomes
Smart Packaging – Gain Confidence in Clinical Trial Outcomes Evaluating and onboarding new vendors to implement a new technology can be daunting. The team at Patheon pharma services has...

Infographic
Four special fill/finish considerations for vaccine production
Once your vaccine is ready for production, there are critical considerations needed in regard to the handling and process for fill/finish. The right choices can help ensure the stability and purity of your vaccine as well as limit unnecessary waste and cost. View the infographic for details on best practices for fill/finish.

Fact Sheet
cGMP Cell therapy manufacturing Capabilities
cGMP Cell therapy manufacturing Capabilities The cell and gene therapy market is experiencing accelerated market approval opportunities, record breaking investments, robust therapeutic pipelines, and positive clinical outcomes – all...

Fact Sheet
Advanced therapy supply chain solutions
From customized packaging and labeling to secure cold chain storage of patient samples, drugs and cell lines, Thermo Fisher Scientific delivers unparalleled supply chain management solutions to serve the...

Infographic
Best practices in viral vector analytical characterization
Best practices in viral vector analytical characterization Characterizing viral vector products and associated impurities is critical to ensuring the safety and efficacy of viral vector therapies. View the following...

Article
Prepping for commercialization through supply chain logistics
Read the following article for insights on common supply chain challenges and considerations when preparing for commercialization of cell and gene therapy products.

Case Study
Consolidating the distribution approach for global clinical trials
Consolidating the distribution approach for global clinical trials A global top-ten pharmaceutical manufacturer needed help improving their distribution strategy. Read this case study to learn how Thermo Fisher Scientific,...

Article
EU Clinical Trial Regulation 2022 –Impact on Regulatory, Labeling & QP
A panel of Thermo Fisher Scientific experts discuss the implications of the new EU Clinical Trial Regulation for pharmaceutical companies and their research and commercial partners

Blog post
Moving from vials to prefilled syringes for vaccines: Three key success factors
As pharmaceutical companies become more patient-centric and self-administration of injectable drugs continues to increase, the market for drug products in prefilled syringes is forecast to grow, reaching $9.53 billion by 2026.
4 minute read

Infographic
Decision point: Build vs. buy continuous manufacturing
Continuous manufacturing is quickly gaining momentum and adoption within the industry due to its cost-saving benefits. Read more to evaluate which is the right option for your company.

Infographic
Five Ways to Get to IND/IMPD Faster
The road to IND/IMPD isn’t always easy. Balancing speed, risk, and future needs is a challenge. So how do you get to IND/IMPD faster without sacrificing quality and future commercialization goals? Check out this infographic to see suggestions from our experts about accelerating and optimizing your early development process.

Infographic
A Tale of Two CDMOs: Which Choice Will Your Biopharma Make?
Today’s growing biologics challenges make the choice of a CDMO critical. You need a trusted and experienced partner from DNA sequence to commercial launch and at each checkpoint along the way.

Infographic
CDMO Timebombs: Five Ways the Wrong Partner Can Lead to Trouble Down the Road
In the evolving biopharmaceutical industry, there is one thing you can count on: Unpredictability. It is in the nature of large molecules generating complex biological activity as scientists seek the right combination of purity, potency, safety and stability – from cell lines to commercialization.

Infographic
Find Your Dream Dose Form: Match-Making Your Brand to the Right Innovation
Managing the lifecycle of your over-the-counter (OTC) drug brand means expanding shelf space, generating premium pricing, and continually innovating in order to win market share from competitors. Download this infographic to meet five high-quality, increasingly popular technologies that may hold the key to your brand’s healthy growth and greater differentiation.

Infographic
Small Pharma, Big Opportunity: Should CDMO Partner Size Influence Selection for your Large Molecule Project?
Overview: With resource constraints and process development challenges within early development, new & emerging companies are often left with the difficult decision on whether to select a small or large CDMO for their steriles drug product. This critical decision not only has implications within early development , but also with scale-up and commercialization.

Infographic
Delivered with speed and service
An overview of our EU central pharmacy solution to support decentralized clinical trials in the EU – a step-by-step approach.

Webinar
Advanced therapies and large molecule investments
Global expansions in development and manufacturing of biologics, cell & gene therapies, and drug products and expansion of clinical supply-chain capabilities

Webinar
Amorphous Dispersion Drug Formulations: A Modern Simulation-Based Approach
Experts explain how to use a structured combination of materials science and molecular modeling to develop a rational formulation design for amorphous dispersions with reduced empiricism.

Webinar
Anticipating the Formulation Challenges of Complex Molecules
Formulation problems are arising with greater frequency, delaying development, and burdening developers with unanticipated and heavy costs.

Webinar
Building a robust FIH biologics regulatory CMC package
When you enter the clinical trial stage of your molecule’s development, you must prepare a dossier to support conducting the clinical research.

Webinar
Bulletproof Your Supply Chain: Hope for the Best, Prepare for the Worst
Incorporating a well-thought out contingency plan is critical when developing a clinical and commercial supply chain.

Webinar
Case Study: Curbing the Opioid Epidemic with Pacira Pharmaceuticals
View this webcast to hear how Pacira is utilizing Patheon’s condominium manufacturing model to manufacture EXPAREL.

Webinar
Challenge Accepted: Biopharma Puts COVID-19 in Its Pipeline
Numerous players are pitching into the fight against COVID-19, with pharma and biotech companies, governments, academic institutions, nonprofits and others working on tests to diagnose the disease and developing vaccines to prevent it.

Webinar
Challenges and practical solutions for changing over to pre-filled syringes for parenteral drug
It’s not uncommon for companies who launch commercial drug products in the pre-filled syringe format to do so after initially using other product formats such as vials for the development and clinical trial phases.

Webinar
Challenges in the Development of Complex Small-Molecule Drugs
Learn the many pieces to the development puzzle of complex small-molecule drugs when taking a new chemical entity to market and potential challenges facing a complex molecule while moving through development.

Webinar
China: Opportunities & high growth potential for global and domestic pharma
How pharma companies in China can leverage the experience of the global market

Webinar
Choosing the Best Biomanufacturing Strategy: The Value of a Multi-Pronged Approach
Hear industry experts discuss the benefits of having a flexible, multi-pronged approach to biomanufacturing, along with the pros and cons of outsourcing versus staying in-house for biologics drug-substance manufacturing.

Webinar
Choosing the Right CDMO for Late Phase Clinical Trials
Gain insight into the importance of looking for a CDMO partner with extensive experience in the parenteral arena, as products move through clinical phases and toward commercialization.

Webinar
Comparator Strategic Sourcing Solutions
Join the webinar by Natalia Kozik, Program Manager, Comparator Sourcing to learn about Thermo Fisher Scientific’s comparator sourcing capabilities.

Webinar
Conventional vs. Unconventional Spray Drying Strategies: Development to Commercialization
Industry experts from across our global network discuss conventional and unconventional spray drying strategies to address bioavailability or crystallization challenges.

Webinar
COVID-19 Threatens Pharma’s Global Supply Chain. What Next?
Hear experts discuss how to navigate the current global supply chain challenges and what improvements Big Pharma and biotech firms should make from the COVID-19 outbreak moving forward.

Webinar
Developments and Opportunities in Continuous Manufacturing
Ensures greater flexibility of supply

Webinar
Does Your Clinical Trial Design Satisfy the Needs of your Patients?
While patient-centric design increases convenience and provides an uninterrupted flow of medication for the patient, it can also positively impact trial enrollment and retention for Sponsors.

Webinar
Driving Supply Chain Productivity in Cell Therapy Clinical Trials
Learn about identifying risks in your cell or gene therapy clinical supply chain, mitigate risk and set yourself up for commercial success.

Webinar
Ensure the Scalability of Your Molecule with a Science-Driven, Phase-Appropriate Approach
Hear experts evaluate how development teams can balance molecule needs and mitigate risks by applying phase-appropriate, science-driven formulation and design principles.

Webinar
Essential up-front planning for your clinical trial
Early, upfront planning of your clinical supply chain is critical to ensuring programs effectively balance risk and cost.

Webinar
EU Clinical Trial Regulation 2022 – Impact on regulatory, labeling and QP
Navigating the new regulatory changes is vital for successfully progressing complex clinical trials in the EU market and ensuring the timely supply of your Investigational Medicinal Products (IMPs).

Webinar
Flexible Solutions to Speed Your Molecules to Phase I Clinical Trials
Watch this webcast to learn how to address early clinical development challenges, and how Thermo Fisher Scientific’s Quick to Clinic™ for Oral Solid Dose can help.

Webinar
From Patient Adherence to Manufacturing Ease—Why Softgels Make Sense for Rx
When it comes to prescription drugs, softgels are not often top of mind for formulators.

Webinar
Getting from R&D to IND – Pitfalls to avoid and how to succeed
How integrated solutions can optimize and speed up the entire DNA to IND workflow and why they’re an effective way to go from an R&D lab to the clinic.

Webinar
Getting your global, small molecule CMC regulatory strategy right from the start
Drug development is dynamic and engages CMC regulatory input at each step.

Webinar
Highly potent strategies from early development to commercialization
Pharmaceutical R&D is focusing on developing more specialized drugs, resulting in increasingly more potent APIs.

Webinar
How an integrated global network of technical, quality and customer engagement teams can support the drug development journey for biologics
Expertise to drive the decisions in the right way to avoid rework and wasting of time.

Webinar
Importance of a one-stop-shop for clinical packaging and labeling in a GMP environment
Join the webinar by Robert Scarth, General Manager, Global Label Services, to learn importance of a one-stop-shop for clinical packaging and labeling in a GMP environment.

Webinar
Innovative Solutions to Bring Medicines to Patients Faster
Watch this webinar to learn about Thermo Fisher Scientific’s $800 million investments in biologics, cell and gene therapy, and drug product capabilities to help biopharma and pharma companies of all sizes accelerate commercialization and bring medicines to patients faster.

Webinar
ISPE Virtual Inspections: Navigating the New Paradigm
Understanding the regulatory landscape for biologics is essential.

Webinar
Leveraging Infrastructure Investments and Innovation to Accelerate Biologics Development
This webinar showcases how Thermo Fisher Scientific is leveraging infrastructure investments and innovation to deliver platform and bespoke solutions meant to accelerate biologics development.

Webinar
Post Pandemic Planning: What Will Change in Early-Phase Drug Development?
Watch as our early development experts discuss the impact of COVID-19 on sponsor/CDMO relationships moving forward, how Thermo Fisher Scientific has maintained normal CDMO operations and plans to shift to meet new needs and more.

Webinar
Post-Pandemic Planning: What Will Change in the Clinical Supply Chain?
Watch as our clinical supply experts discuss the changes the clinical supply chain will face as a lasting result of COVID-19, how innovations from clinical service organizations such as Thermo Fisher will help move trials forward, and more.

Webinar
Practical Implementation of Innovative and Scalable Technologies to Accelerate Biologics Development and Commercialization: A CDMO Perspective
Watch this webinar to learn how new and innovative technologies and best in class manufacturing capabilities can help alleviate challenges in Biologics development.

Webinar
Process Characterization: Ready For The FDA?
Impact of process characterization and process validation/PPQ campaigns.

Webinar
Process Characterization: Ready for the FDA/EU ICH guidelines
There is a common view among biopharmaceutical regulatory agencies that process validation is requirement for all commercial products.

Webinar
QP Release for drug products in Europe
Understanding regulatory legislation is vital for successfully navigating the complex global clinical trials market and ensuring the timely supply of your Investigational Medicinal Products (IMPs).

Webinar
Regulatory uncertainties: Continuous manufacturing
One of the biggest barriers manufacturers point to when it comes to the lack of widespread adoption of continuous manufacturing is uncertainty.

Webinar
So Many Choices: What’s the Right Biomanufacturing Strategy for Me?
Watch excerpts from this Patheon-sponsored super session at BIO International, as Steve Lam presents, “So Many Choices: What’s the Right Biomanufacturing Strategy for Me?”

Webinar
Taking a More Informed Approach to Solubility Enhancement
Patheon Solubility Enhancement Services approaches BCS II drug substance solubility issues a fundamentally different way.

Webinar
Taking An Informed Approach To Technology Selection To Address Solubility Challenges In Early Development
As the need for more targeted drug therapies has increased and drug development has become more complex, the industry has seen a corresponding rise in the number of molecules with low solubility challenges.

Webinar
Technology transfers: Best practices for optimizing success and mitigating risk
When working with the right partner and network, transferring production between sites allows companies to reap a variety of strategic advantages.

Webinar
Transportation Solutions for Cell and Gene Therapy
From the basics such as service level expectations for couriers versus integrators, to how to evaluate domestic and international lanes, and finally—how to balance risk and cost, this webinar will help you understand how to start tackling your clinical supply chain.

Webinar
Vaccine Development: Strategic Approach & Response During Pandemics
Over the past decade, outbreaks of H1N1 influenza, Sars-Cov, Ebola, and MERS have sparked ongoing conversations about strategic approaches and responses to pandemics and epidemics.

Webinar
Your Clinical Results Look Promising, but are You Ready for Launch? How to Build a Robust Packaging Strategy for Rapid Commercialization
Now that you are starting to see promising clinical results, the focus quickly shifts to commercialization and the many challenges that come from planning a pharmaceutical packaging strategy.

Webinar
QP release in the EU in 2022 and beyond: Your questions answered
In Europe, and in the UK, the Qualified Person (QP) plays a crucial role in bringing safe and timely products to the market and/or supplying them to the clinic.

Webinar
Establishing a correctly characterized molecule in early drug development
The physical properties of a solid active pharmaceutical ingredient (API) largely determine the crystallization process used for isolation, purification and form control, and also guide the development of an effective formulation.

Webinar
Top 5 Considerations When Looking to Outsource to a Biomanufacturing Partner
Join Paul Jorjorian for a 20 minute “Ask the Expert” webcast with BioProcess International.

Webinar
Regulatory and cost implications for switching injectable delivery formats
Since there are various options for injectable formats, it’s not an uncommon practice to launch a large molecule in one format and then later decide to switch the delivery format.

Webinar
Addressing scalability challenges in the development and manufacture of gene therapies
Viral Vectors have been approved for use in gene therapies, gene modified cell therapies, and vaccines to address rare disease, cancer and public health issues

Webinar
Addressing technical challenges in development drug products: A CDMO perspective
Throughout the development of small and large molecule drug products, there are various demands and technical challenges that can cause lengthy and costly delays.

Webinar
Building viral vector capacity and capabilities to realise the promise of gene therapies
Challenges and opportunities of bringing viral vector products to market.

Webinar
Cell and gene therapy manufacturing in a post-covid world
In this webinar, our speakers will share their expertise in cell and gene therapy manufacturing, and discuss the future of the industry, and how developers can adapt to this new paradigm.

Webinar
Common Softgel Myths Debunked
Watch as Kaspar van den Dries, Senior Director of Science and Innovation at Thermo Fisher Scientific, deconstructs common myths surrounding softgels for prescription formulation.

Webinar
Continuous manufacturing: Drug development workflow and benefits
Overview the economic and quality benefits of oral solid dose continuous manufacturing for commercial drug products and understand how leveraging continuous manufacturing in development can allow greater assurance of quality as well as scale up efficiencies.

Webinar
Drug repurposing trends—A CDMO perspective
Overview the trends and CDMO perspectives on the low-risk, cost effective drug repurposing strategy to bring off-patent generics, clinical, shelved drugs or combination drugs to treat different indications and faster to market.

Webinar
Early Stage Development for Solid Dose Products
Review challenges experienced in early stage development of solid oral dosage forms and the implications of these challenges on a development program.

Webinar
Executive Research: Impact of Incorrect Forecasts on New Product Launches
Hear the results of the ORC International study, including forecasting processes, key issues that arise from inaccurate forecasts, and how forecasting needs will change over the next few years due to evolving organizational needs.

Webinar
FDA Accelerated Approval Pathways for Cell and Gene Therapy Products
Understanding the regulatory landscape is essential. It is changing as fast as the industry is changing.

Webinar
Fixed dose combination drug development: Designing a lifecycle strategy with agility & speed
The development of fixed dose combinations and drug repurposing projects are commonly adopted lifecycle management strategies for the enhancement of individual drugs.

Webinar
How Adaptable Manufacturing Models are Paving a Steady Path into an Unpredictable Future
In a short 20 minutes, you can learn about manufacturing trends, adaptable manufacturing models, and take a tour of Pacira’s condominium manufacturing facility at Patheon’s Swindon location.

Webinar
How Well Should You Understand Your Crystallization Process (and Solid-State Properties)?
Crystallization is the most common unit operation for isolation and purification of solid intermediates and active pharmaceutical ingredients (APIs).

Webinar
Identifying and Solving Scale-up Challenges in the Synthesis and Formulation of Small-Molecule APIs
Join pharma services expert, Dr. Peter Poechlauer, as he examines the process of identifying and solving scale-up challenges in the synthesis and formulation of small-molecule APIs.

Webinar
Manufacturing Innovation: The Case for Continuous Manufacturing
During this on-demand webinar, hear from industry expert Doug Hausner compare and contrast continuous manufacturing and traditional batch manufacturing.

Webinar
mRNA vaccines, trends, technologies and supply chain
Challenges and considerations for achieving a robust global vaccine supply chain.

Webinar
Navigating Decision Points to Fast-Track Commercialisation
Understanding the risks, requirements and challenges of bringing clinical products through validation and to the market are vital for the success of your large molecule project.

Webinar
Orphan Drugs: Balancing Financial Incentives & Complex Challenges
Orphan Drugs are notorious for their high costs and risk factors, which is attributed to smaller patient pools and higher development and launch costs.

Webinar
Phase-Appropriate Formulation Development & Manufacturing for Parenteral Dosage Forms
Watch this webinar to discover the criteria that pharmaceutical development teams should consider when ensuring their parenteral dosage form is prepared to scale to late stage and commercial.

Webinar
Preparing for an evolving regulatory landscape to successfully commercialize cell and gene therapies
Cell and gene therapies are progressing through clinical trials and driving towards commercialization at a rapid pace.

Webinar
Shortening the timeline to first in human (FIH) clinical dosage form for oral / injectable delivery
In recent years, with more clinical candidates being explored for niche indications, orphan drugs, and for indications with a rapid clinical end point, the pathway to the later clinical phase is short and there is not enough time to perform bridging studies.

Webinar
Simplify Your Supply Chain in the Race to Phase 1
In this webinar, experts Kevin Kane, PhD and Iain McGroarty, PhD discuss how a simplified supply chain may help increase efficiency and reduce risk as your move to your Phase I milestone.

Webinar
The Advantages of Employing a Lipid-Based Formulation Process in Early Development
In this webinar, experts will explain the benefits of considering lipid formulation early in the development process and how a structured screening and development approach can assist in overcoming potential barriers associated with lipid formulations.

Webinar
Trends and Challenges in Outsourced Oral Solid Dosage Forms
As drug companies have fewer and fewer new compounds entering their R&D pipelines, outsourcing of development and manufacturing activities for oral solid dosage forms and sterile forms is on the rise.

Webinar
What to Look For in a Solubility Enhancement Vendor
Patheon’s Kaspar Van den Dries sits down with Pharmaceutical Technology Magazine to discuss which crucial characteristics you should look for when researching and selecting a solubility enhancement vendor.

Webinar
Why are Lipid Formulations Commonly Used to Enhance Bioavailability?
Join Thermo Fisher Scientific’s Kaspar van den Dries and Helena Teles as they discuss potential mechanisms of increased absorption with lipid formulations, the appropriate screening tools used during the development approach of these formulations, and the subsequent steps to scale up and industrialize them.

Webinar
Why Did Thermo Fisher Scientific Acquire Patheon?
In August 2017, Thermo Fisher Scientific completed the acquisition of Patheon, creating the world’s most comprehensive and sophisticated end-to-end CDMO partner.

Webinar
Smart packaging: The power of adherence data
The value of individual data points that are passively captured at each patient medication dosing event are often overshadowed.

Webinar
A toxicologist’s viewpoint on developing oncology drug products
With oncology growth rates on the rise, pharma companies are pressured to develop and manufacture life-saving therapies with speed and agility.

Webinar
Parenteral drug market: Meeting escalating challenges
Listen to this on-demand webinar to hear from industry experts, Christy Eatmon and Peter Shapiro.

eBook
Total Transportation Management
The transportation of life science shipments has become increasingly more complex. Learn how to overcome challenges and avoid the high cost of failure.

eBook
The Challenge of Keeping Cool: End-to-End Temperature Management for the Clinical Supply Chain
Learn about managing the storage and transportation environment, what the regulatory definitions are, and planning for end-to-end temperature management.

eBook
Smooth Transition: What Specialty Drug Manufacturers Should Know About Bridging the Gap from Clinical-to-Commercial Packaging
As a steady stream of specialty drugs enter the marketplace, pharmaceutical companies are seeking new supply chain solutions to ensure that an uninterrupted supply of these breakthrough treatments reach patients. One solution for the challenges of packaging specialty products is low- or small volume commercial packaging.

eBook
Reasons for Building an Approved Phrase Library
Learn how an approved phrase library can reduce label timelines, improve quality, and assure regulatory compliance.

eBook
Managing Temperature Excursions: Key Pointers That Must Be Addressed
Discover what to do when a temperature excursion occurs and get summary recommendations on how to handle cold chain or temperature sensitive IMP across the supply chain.

eBook
Importer of Record Frequently Asked Questions
Get answers to frequently asked Importer of Record questions, a checklist of roles and responsibilities and a glossary of terms.

eBook
Evaluating and Qualifying Temperature Managed Shippers
Discover the top 5 questions to ask when evaluating shipper options and the many benefits of return and re-use programs. Plus, get the results of a case study and pilot program.

eBook
Cold Chain Qualification: 5 Questions You Must Ask When Shipping Biologics
Download the eBook to learn about the different types of considerations when shipping biologics.

eBook
Cold Chain Industry Trends
Take a look at the importance of a robust supply chain and get planning recommendations for pharma companies scaling up to global vaccine trials.

eBook
Clinical Trial Packaging: Innovative, Flexible and Time-Sensitive Solutions
Clinical trial packaging solutions can be as diverse as the medications they protect. Learn how our clinical study sponsors were able to implement a packaging design that scaled through all phases of the trial while reducing time and waste.

eBook
Clinical Trial Packaging Solutions: Balancing Cost, Time and Quality
Explore critical factors in the packaging design process that can deliver the best possible outcome for your clinical study and, ultimately, the patient.

eBook
Clinical Trial Packaging: Smart Choices Can Trim Timelines
Get important insights to help guide the development of your packaging strategy to streamline study timelines and increase the likelihood of success.

eBook
Beyond Procurement: Taking a Strategic Approach to Comparator Drug Sourcing
Sourcing a comparator drug with the highest quality and with a short lead-time can be challenging. Find out how a strategic approach can help.

eBook
Ancillary Management: Keep Your Clinical Trial on Track
Learn the industry trends that are driving increased focus on a successful ancillary strategy and important considerations to develop a comprehensive strategy that minimizes risk.

eBook
Top 5 Questions Asked About Pre-Filled Syringes
Asking key questions of your prospective supplier will help you make a better selection and design a better solution. Find out what they are and why they're important.

eBook
Future Forward: How your outsourcing strategy shapes your development strategy
Once you decide if you are going to outsource drug product manufacturing, it's important to start building a robust strategy that helps you move through each phase with agility and speed. Part of that strategy entails a key decision point. Should you outsource your development program to: 1. Single vendor 2. Several vendors

eBook
The 5 pitfalls of API development
No matter where you are in the API development life cycle, early planning makes a big difference. Download this slide module to learn about common pitfalls in each stage of development and how the right outsourcing partner can help you avoid them.

eBook
Growing Your Biopharma: Ten Questions You’re Likely to Face From Investors—And How to Respond to Them
There are many important considerations to address in building your new drug program. Without a doubt, one of the most critical is funding to get you to your next milestone. As you grapple with funding issues, it can be useful to consider the point of view of a potential investor. Investors are betting on you to manage development effectively and to move quickly.

eBook
There May Be Dragons: Mapping 7 New & Emerging Pharma Development Risks
You have to think about things like technology transfers, shipping and logistics, as well as the increasingly complicated regulatory expectations across countries. Large pharma companies often have the staff and experience to manage and move drug products and ancillaries within and across borders, but new and emerging companies tend to run lean and be scientifically focused. As a result, you may not have the resources or expertise to coordinate development programs across multiple sites, geographies or vendors. This eBook is designed to help you map seven key risk areas in drug development and clinical services—and build a plan to overcome them.

eBook
No Place like Home: How to Make Decentralized Clinical Trials a Win for Patients, Sponsors & Investigators
Download this eBook to learn about the integral role Direct-to-Patient Services play in the execution of decentralized trials and how sponsors can maximize these benefits to improve drug development.

eBook
Validation or Qualification – What’s the difference?
For those studying, manufacturing, or experimenting with delicate pharmaceuticals and biological samples, the importance of sample storage and accurate temperature monitoring is well known. From a qualification and validation perspective, the equipment and processes used for shipping, storing (whether refrigerated storage or ultra-cold storage), and other services must comply with the products’ requirements throughout the chain of custody. The terms “validation” and “qualification” are used interchangeably and loosely at times.

eBook
Defense in Depth – Off-Site Storage of Biological Specimens and Biopharmaceuticals for Risk Mitigation
The management of risk is part of all business operations, but to commercial biobanks, clinical research institutes, biotech, and pharmaceutical companies, risk mitigation is a critical element of day-to-day operations. The costly nature of irreplaceable samples/cell lines and high value products such as cell-based drugs, and biological active pharmaceutical ingredient (Bio-API) dictates planning for the full continuum of risk. The best solution is frequently off-site storage.

eBook
Thinking Outside the Freezer: DNA and RNA Storage
Download this ebook to learn alternative solutions for DNA and RNA storage options.

eBook
Safely Transporting Medicines to Patient: De-Risking the Supply Chain
Download this eBook to view the survey results and accompanying analysis about what it means to pick the best pharmaceutical logistics partner.

eBook
Cell Therapy Logistics: Advanced Therapy
Overview key considerations for developing a successful logistics strategy for the management of cell-based material.

eBook
Flexibility Enables Managing Large Leaflet on Automatic Packaging Line
A large multinational pharmaceutical company asked the Thermo Fisher Scientific Ferentino site to fix an issue that resulted from a change request to increase the size of the product information leaflets. These leaflets were important and required for regulatory and marketing reasons. Download the case study to learn more.

eBook
Cold Chain Shipping Considerations
Overview cold chain shipping considerations, examine different temperature controlled packaging options and learn the advantages/disadvantages of various coolants.

eBook
Using an Approved Phrase Library
Find out how to avoid delays in translating and approving clinical labels that can prevent clinical trials from starting on time and threaten to derail development timelines.

Case study
Collegium Pharmaceutical’s Approach to Abuse Deterrence Breaks New Ground
The opioid abuse epidemic is a serious public health crisis that demands action on the part of many stakeholders. While abuse-deterrent opioids will not solve the opioid abuse epidemic alone, they play a critical role in the fight against it. While Collegium had come up with an innovative new approach to abuse deterrent formulations, it did not have the capacity or resources to manufacture its drug on a commercial scale.

Brochure
Softgel Technologies Overview
Thermo Fisher brings value to pharmaceutical and consumer health care companies through ideation sessions and flexible business models that can: Develop product Proof of Concept to confirm market interest,...
Brochure
Biopharma Capabilities Overview
Learn more about our integrated offering for biopharma organizations. Built on a foundation of quality systems and commitment to continuous improvement, we help you achieve success at every milestone.

Brochure
Rx softgel solutions: Find the right dosage form to suit your molecule
Choosing the right partner to develop your Rx softgel drug can be a difficult decision. Whether your product has solubility issues—like 70% of drugs in development—or requires abuse-deterrent technology,...

Brochure
Small molecule API solution: Taking a big picture approach
With competitive options for API outsourcing in all geographical regions, it can be difficult to choose the right partner for your project, regardless of its complexity. Thermo Fisher Scientific...

Brochure
Steriles drug development and manufacturing: Flexibility and optimization
The rapid 10 percent growth of sterile drug product over the past five years has resulted in the need for capacity and innovative solutions within development and manufacturing. Navigating...

Brochure
Developing a CMC and regulatory roadmap for your molecule’s lifecycle
Getting your strategy right from the start of your molecule’s journey can help save time and money as you advance through each phase and proceed to commercialization.

Brochure
Oral Solid Dose for Oncology
Thermo Fisher Scientific offers robust drug substance, drug product, and clinical trial supply solutions to scale your small molecule all under one roof.

Case study
Development of Qualified Cold Chain Solution for Vaccine Transport to Uganda
A government contractor required the development of a repeatable and effective pack out which could maintain steady temperature of 2°-8° C (35°-46° F) during transit for the transportation of vaccines (varying payloads) which were shipped to Uganda as part of a study. This was quite challenging given the logistical challenges that exist given the remoteness in access.

Case study
Clinical Supply Optimization: Mid-Sized Pharma Embraces Forecasting
With no solid demand planning due to understaffing, a mid-size pharmaceutical company ran into serious trouble with stockouts and incorrect drug shipments that threatened to jeopardize its clinical trial program. Learn how Thermo Fisher quickly assessed the issue and reconfigured the interactive response technology to start producing accurate forecasting statistics, thereby saving the program.

Case study
Packaging Design Provides Time and Cost-Efficiencies for a Growing Biopharma Company
Learn more about the process of designing a blistering package project that delivered an optimal, cost-effective solution for a growing biopharma.

Case study
Automation Slashes Turnaround Time for Clinical Trial Labeling
Find out how a radical reduction in the time it takes to produce clinical trial labels was realized for a multinational trial.

Case study
Cold Chain Reusable Shipper Reduces Risk and Waste
Learn how a multinational pharmaceutical company found itself face-to-face with a sustainability challenge and turned to a new shipping solution, reusable shippers, to help them reduce their waste and carbon footprint.

Case study
Clinical Packaging: Process Overhaul Cuts Cycle time by 55%
Learn how lean manufacturing techniques, label modifications and operational improvements helped support clinical trials of a blockbuster oncology drug.

Case study
Clinical Supply Global Optimization
Read this case study to gain insight into how to smooth the execution of your distribution strategy and ensure all your patients have the right medication on time.

Case study
How a global network enabled distribution of an oncology drug to 60,000 patients in 35 countries every month
Angie will never forget the day she got a call from a client with a packaging and logistics challenge of global proportions. They needed help figuring out how to get an oncology drug labeled for 60,000 patients in 35 countries every month. And they needed that solution to fit in a four-week cycle. Read the case study to learn how the team reduced the cycle time in half.

Case study
How overcoming barriers ensured on-time delivery of a life-changing medication
A new drug made in Canada. Shipped to Mexico. Now needed in Brazil. If the client didn’t make this launch, the region would have lost all access to this medication. Download this case study and see how one team’s experience cracked the code.

Case study
Delivering Clinical Trial Medications Direct-to-Patient
Download this case study to learn how Thermo Fisher Scientific was able to provide Direct-to-Patient services to meet a critical need.

Case study
Global Ultra-Cold Clinical Trial Logistics
The Challenge: A new United States-based biopharma working on its first cell therapy-based treatment had expanded the scope of their trial and extended its patient pool to include a patient in Beijing, China.

Case study
Expedited Import of Zika Virus Test Kits Enable Vital Research
Given the rapid and widespread outbreak of this virus, researchers were urgently working towards a solution. In a significant breakthrough, a European-based manufacturer had successfully developed a test kit that enabled serological detection of the Zika virus. Getting these kits into the labs of the United States researchers became a critical priority.

Case study
Direct to Representative Sample Distribution Services
Learn how we helped a manufacturing company design an efficient sample distribution program to minimize product loss, while ensuring full compliance with industry regulations.

Case study
Managing Clinical Ancillary Supplies at a Global Scale
Follow a biopharmaceutical company entering a Phase III trial that included 45 countries and an enrollment target of 4,000 patients in a randomized, double-blind, placebo-controlled study.

Case study
How an emergency IND was able to reach a patient in under 12 hours
It’s Saturday. A six-day-old baby needs emergency medication. You need a team that’s prepared for situations like this to turn two and a half days of logistics into just 12 hours. Download this case study to learn how Holli and her team made it happen.

Case study
We work together to meet global demand for COVID-19 vaccines
Learn what led to Thermo Fisher Scientific looking for a bespoke packaging solution, one that enabled work to be carried out within the low temperatures and specific timescales needed

Brochure
Leading Viral Vector CDMO Services for Cell and Gene Therapies
With 14+ years of experience in viral vector services, Patheon is your end-to-end viral vector CDMO partner from process and analytical development to clinical and commercial supply of viral...

Brochure
Global clinical supply solutions for every trial delivering the right drug to the right patient, on-time, in-full, and without compromise
In the highly competitive, new drug development market, biopharma companies face a myriad of challenges—from balancing cost, time, and quality, to delivering the best possible outcome for their...

Brochure
OTC softgel solutions: Give your branded product a competitive advantage
Thermo Fisher Scientific can offer innovative ideas on brand expansions via our own softgel technologies that can give your branded product a competitive advantage—helping you stand out to consumers....

Brochure
5 Ways Your Viral Vector Manufacturing Partner Should Make You Feel
In viral vector manufacturing for cell and gene therapies, success requires a level of commitment and dedication that makes...

Brochure
From Molecule to Medicine
Patheon, part of Thermo Fisher Scientific, is transforming the way pharmaceuticals are made with a simplified, end-to-end supply chain for pharmaceutical and biopharmaceutical companies of all sizes. Learn how.

Brochure
Pharma Services EMEA network, integrated network from molecule to medicine
Our objective is to help speed your molecule through early phase trials and prepare you for commercial success, faster. In this brochure, find out more about our dedication and...

Case study
Fighting The Opioid Epidemic: How Grünenthal’s Abuse-Deterrent Technology Contributes
Learn how Grünenthal overcame various manufacturing challenges and utilized the expertise of a CDMO to develop its abuse-deterrent formulation, INTAC®.

Case study
Using Quality By Design For Process Development And Scale-Up Of A Novel ALS Drug Product
Learn how our stepwise approach to Quality by Design (QbD) became integral to the success of the Amylyx Pharmaceuticals AMX-0035 campaign.

Case study
Clinical supply optimization enhanced services – process improvement reduces study startup phase by 40 percent and drives improved supply chain execution
Clinical supply optimization enhanced services – process improvement reduces study startup phase by 40 percent and drives improved supply chain

Case study
Clinical supply optimization – investigator supported studies streamlined
The key to a successful program of investigator supported studies is maintaining productive relationships with the physicians at the clinical sites.

Case study
Clinical supply optimization – enhanced service model saves trial sponsor time on supplies
The vice president of manufacturing for a clinical-stage biopharmaceutical company faced an avalanche of work when a reorganization left him without a clinical supply manager.

Case study
Clinical supply optimization – digging for the true cost of clinical supply
Pharmaceuticals are a small part of this company’s overall business and its team was relatively new to clinical supplies.

Case study
Clinical supply optimization – clarifying the relative risk of change
The clinical team at this top ten pharmaceutical company struggled with the delicate balancing act between controlling the costs of a major oncology trial and making strategic investments that would pay off in the long term.

Case study
Clinical ancillary supply management – streamlining ancillary supplies and the investigator experience
A top 25 global pharmaceutical company came to Thermo Fisher Scientific requesting support for a complex global program encompassing 15 studies across 53 countries over 1,000 sites and for over 12,000 patients.

Case study
Clinical ancillary management – tackling the complexities of a global diabetes trial
A large manufacturer needed support for their global diabetes trials. The scope of a single trial often included as many as 1,500 to 2,000 patients in 15 to 20 countries across all geographic regions (US, EU, Asia, Middle East).

Case study
An alternative logistics approach realizes cost and performance efficiencies in Asia Pacific
A top major pharmaceutical company with research priorities aligned to significant global health needs had a high number of studies in progress in the Asia Pacific region.

Case study
Accessing patient populations in remote locations presents challenges for ambitious new trial
A top major pharmaceutical company needed guidance on how to run an ambitious clinical trial in the Asia Pacific region involving 10+ countries, 74+ sites with 400+ shipments across the region.

Case study
Total Transportation Management Saves Company $10.2M
Learn how a multinational pharmaceutical company optimized transportation costs, maximized on-time, in-full delivery to all global locations and delivered enhanced site satisfaction.

Case study
The Criticality of API CDMO Selection: Insights from a Client
Small and emerging companies face challenges related to limited funding and resources, such as technical expertise and capacity, making it imperative they find a competent partner to support them. Learn why 4SC selected Thermo Fisher Scientific Pharma Services as their CDMO partner to develop its lead asset, resminostat.

Case study
The Critical Importance of Comparator Temperature Excursion Management: A Robust Quality Assurance Process
Learn how Thermo Fisher helped two clients to overcome comparator sourcing challenges and avoid costly delays in their clinical trials.

Case study
Not Just A Vendor, But Part Of The Team
Learn how the Clinical Label Services team helped an emerging biotechnology company reduce cycle times and ease the burden of lengthy clinical timelines and unhappy sites.

Case study
Meeting Milestones and Patient Needs Through Expedited Delivery of Phase I Materials
Learn how Reneo Pharmaceuticals got to clinic in 14 weeks from delivery of their API with the Quick to Clinic™ for Oral Solid Dose program.

Case study
How to Select the Best Courier Around the Globe
Get an in-depth look at the clinical trial challenges faced by a world leader in biotechnology and the actions taken to realize surprising results.

Case study
How Pacira Pharma is Working To Help Curb The Opioid Epidemic
Poorly managed postoperative pain can have a significant impact on a patient’s recovery. Consequently, more patients relying in opioids furthers compounds the opioid epidemic. This case study discusses how Patheon and Pacira Pharma partnered to help curb this epidemic.

Case study
How a Best-Practice Strategy for Sourcing Ancillary Materials Reduced Risk, Spending and Workload in a Global Diabetes Trial
Learn how a sound strategy and solid execution plan enabled this company to overcome the challenges they faced to ensure that their patients received the ancillary products they needed.

Case study
High Precision Syringe Labeling
Discover how high precision labeling helped a customer who had to blind their syringe against commercial drug product by applying a label containing a dosing scale to syringes of varying lengths.

Case study
Flexibility Enables Managing Large Leaflet on Automatic Packaging Line
A large multinational pharmaceutical company asked the Thermo Fisher Scientific Ferentino site to fix an issue that resulted from a change request to increase the size of the product information leaflets. These leaflets were important and required for regulatory and marketing reasons. Download the case study to learn more.

Case study
Early Licensure of a Breakthrough Cancer Drug Gives Hope to Patients
This compelling case study outlines how a multinational pharmaceutical company worked with a cross-functional Fisher Clinical ServicesSM team to deliver clinical supplies to treat an aggressive form of skin cancer, Merkel cell carcinoma.

Video
The Medicine Maker, Chapter 1: Understanding Industry Shifts
Video Webinar with The Medicine Maker, Chapter 1: Understanding Industry Shifts.

Video
The Medicine Maker, Chapter 2: Overcoming Commercial Launch Challenges
Video Webinar with The Medicine Maker, Chapter 2: Overcoming Commercial Launch Challenges.

Video
The Medicine Maker, Chapter 3: Identifying Volatile Forecast Variables
Video Webinar with The Medicine Maker, Chapter 3: Identifying Volatile Forecast Variables.

Video
The Medicine Maker, Chapter 4: Measuring Forecast Accuracy
Video Webinar with The Medicine Maker, Chapter 4: Measuring Forecast Accuracy.

Video
The Medicine Maker, Chapter 5: Planning for Insourcing or Outsourcing
Video Webinar with The Medicine Maker, Chapter 5: Planning for Insourcing or Outsourcing.

Video
The Medicine Maker, Chapter 6: Leading Industry Transformation
Video Webinar with The Medicine Maker, Chapter 6: Leading Industry Transformation.

Video
Clinical and Commercial Manufacturing for Oral Solid Dose in Greenville, North Carolina
Clinical and Commercial Manufacturing for Oral Solid Dose in Greenville, North Carolina.

Video
Clinical and Commercial Manufacturing in Brisbane, Australia
Our Brisbane, Australia site is 2014 winner of the ISPE Facility of the Year award and 2015 winner of the Frost and Sullivan APAC CMO of the year award. The site provides scale up and manufacturing services of recombinant proteins and monoclonal antibodies.

Video
Continuous Manufacturing in Patheon’s Greenville, North Carolina Site
We are the first CDMO to build and manufacturing client product on a continuous manufacturing line. The suite, located at the site in Greenville, North Carolina, is custom built to utilize the best equipment available.

Video
Delivered With: A Positive Client Experience
Learn how a global CDMO never loses sight of what’s important to our clients.

Video
Delivered With: Partnership and Passion
Learn why this supply chain executive calls Thermo Fisher Scientific the CDMO partner of choice.

Video
Early Development and Oral Solid Dose in Milton Park, UK
Discover Thermo Fisher Scientific’s formulation and clinical manufacturing site in Milton Park, UK featuring proven expertise in early development and oral solid dose.

Video
Fisher Clinical Services & Patheon: Better Together
Leon Wyszkowski, President, Commercial Operations, discusses the value of Patheon and Fisher Clinical Services joining together to provide integrated solutions for our clients.

Video
Flexible Oral Solid Solutions From Early Development to Commercial Manufacturing
Thermo Fisher Scientific provides a range of flexible oral solid dose solutions that can help you easily address your small molecule’s unique needs and challenges from early development to commercial manufacturing.

Video
Going the Extra Mile is Part of Our DNA
The stakes were high for a client who needed material for a novel drug to in order to proceed to clinical trial. With millions of dollars on the line, Jeff’s team had one shot to develop a process to scale up or risk failing the batch.

Video
How a Small Non-profit Teamed up with a Global Manufacturer to Bring Their ALS Drug to Clinical Trials
Before she lost her own battle to ALS in 2003, Jenifer Estes started Project ALS, raising over $17 million dollars for the non-profit in hopes of a breakthrough in the fight against Lou Gehrig’s disease. As her family continued the quest, in 2019, they got a hit with a new compound that seemed to stop or even reverse motor nerve damage.

Video
How a Viral Vector Got the Boost It Needed to Start Defeating an Incurable Disease
Duchenne’s Muscular Dystrophy is an incurable disease that mostly affects boys. It results from a gene defect that prevents those affected from developing normal muscle structure and function.

Video
How Can Pharma Companies Save up to $45M in Early Drug Development?
Patheon’s Jennifer Therrien discusses findings of a Tufts Center for the Study of Drug Development (CSDD) study that reveals how pharmaceutical companies using a single-source outsourcing partner can achieve a net gain of $45 million in early drug development.

Video
How Embracing Flexibility Creates Success for All
Learn how Whitney took on the challenge of creating more flexibility within existing rigid processes to better meet the needs of her client. Together, they came up with a solution to optimize critical paths forward to expedite getting life-saving therapies to the patients who need them.

Video
How One Split-Second Decision Made a Difference for Thousands of Patients
In the world of drug manufacturing not all life-saving decisions happen in the lab. Sometimes it’s a lot closer to delivery that key moments require fast thinking.

Video
How Speaking a Third Language Helped Win the Race to Market
Several Chinese pharmaceutical companies were competing to be first to market. And second place didn’t matter.

Video
How Spring Break Had to Wait a Little Longer, So a Baby Could Have a Better Shot
After a long night of packing for her family’s spring break vacation, Holli woke up at 4:40 AM Saturday morning. She noticed a text message on her phone.

Video
How Thermo Fisher is Battling COVID-19
We are proud to be on front lines arm-in-arm with our clients, fighting COVID-19. Together, we will win. Every patient matters.

Video
How two college kids took a dorm-room idea all the way to clinical trials.
Watch the video to learn why companies like Amylyx are choosing to partner with Thermo Fisher Scientific for their outsourcing needs.

Video
How Viral Vector Technology Is Rapidly Scaling up to Enable One Miracle After Another
The promise of viral vectors has been pursued for over two decades. But in the last few years, this transcendent technology that’s targeting over 200 diseases has finally started to create real treatments and possible cures.

Video
Made For: Hope for ALS
Learn how Amylyx Pharmaceuticals partners with Thermo Fisher Scientific to bring hope to patients with ALS.

Video
One Bottle of Hope for One Amazing Kid
Meet Gavin and his mom, Nicole. Learn how Gavin’s battle with a brain tumor led him to the bottle of hope that allowed him to be a kid again.

Video
Patheon’s Softgel Expertise
Learn more about the Softgel expertise at Patheon, by Thermo Fisher Scientific.

Video
Single-Use Technology for Biologics Manufacturing in St. Louis, Missouri
Learn about Thermo Fisher Scientific’s expansion of its biologics site in St. Louis, Missouri. The facility will be the single largest single-use technology CDMO in the United States.

Video
Softgel Manufacturing in High Point, NC
Discover how softgels present a viable option to help achieve drug solubilization, allowing enhanced absorption and bioavailability.

Video
Solubility Center of Excellence in Bend, Oregon
Discover Thermo Fisher Scientific’s early development site in Bend, Oregon, featuring proven expertise in solving solubility challenges for clients.

Video
Solved With: Capacity and Compassion
Learn how Thermo Fisher Scientific was able to help this company solve one of their biggest challenges in the drug development process.

Video
Step Beyond with Thermo Fisher Scientific
To serve science, Thermo Fisher Scientific needs to stay ahead of it. To be the world leader in serving science, we need to anticipate customer needs.

Video
Sterile Drug Product Development and Manufacturing In Greenville, North Carolina
Meet the team and tour our Greenville, North Carolina manufacturing facility, which a focus on sterile drug product development and manufacturing.

Video
Sterile Vial and Prefilled Syringe Fill-Finish in Monza, Italy
Take a look inside Thermo Fisher Scientific’s sterile fill-finish manufacturing site in Monza, Italy. The site currently manufactures commercial supply, but has recently invested $50 million in sterile drug development capabilities.

Video
The “Eureka Moment” That Helped a Small Company Make a Big Impact
Tony and the team at Thermo Fisher Scientific understand the needs of small and emerging companies.

Video
The Journey from Molecule to Medicine
It all starts with your discovery. A molecule, small or large, that needs a partner. Let Patheon be that partner.

Video
Thermo Fisher Scientific & CSL Enter Strategic Partnership to Provide Best-in-Class Pharma Services
We are pleased to announce that Thermo Fisher Scientific and global biotechnology company CSL have entered into a strategic partnership to help meet the growing demand for biologic therapies while also accelerating CSL’s broader manufacturing objectives.

Video
Vectoring In — Viral Vector Video Series
Preparing cell and gene therapies for regulatory submission
When preparing to ramp up late stage manufacturing for commercialization, understanding key critical to quality parameters will help prepare your therapy for regulatory submission.

Video
You Inspire Us Every Day
Michel Lagarde, Executive Vice President, Thermo Fisher Scientific, discusses why Pharma Services employees are so passionate about partnering with small and emerging biopharma clients.

Video
Emily and Jessica’s story: Culture of Knowledge Sharing
Learn how Emily and Jessica continued to share knowledge between their teams, helping information reach clients faster to incorporate into clinical trial protocols.

Video
Our Formulation of Heart and Science is the Key to Our Success
At Thermo Fisher Scientific, everything we do is made with the right balance of heart and science. Discover how Angie’s team overcame a compressed timeline for a large global clinical trial with coordination across departments to expedite cycle times.

Video
Quick to Clinic™ for Oral Solid Dose
Learn how Reneo Pharma was able to get to clinic in 14 weeks from delivery of their API.

Brochure
Biologics Overview: Flexible Biomanufacturing Solutions
Your molecule has the power to change lives and shape the future. Patheon is the company that offers you the agility and speed to help you get there ahead...

Brochure
Clinical Trial Supply Solutions
Learn how our global team can meet the needs of every trial regardless of size, phase or therapeutic area.

Brochure
Transportation Solutions for Cell and Gene Therapy Supply Chains
Learn key considerations for evaluating transportation partners for your cell and gene therapy supply chain.

Brochure
Continuous Monitoring Solutions for Cold Chain Logistics
Download this fact sheet to learn about our continuous monitoring solutions for cryogenic shipments.

Case study
Free trade area attracts zone sponsors to India
A top major pharmaceutical company needed guidance on how to run an ambitious clinical trial in the Asia Pacific region involving 10+ countries, 74+ sites with 400+ shipments across the region.

Case study
Expert handling of high-risk clinical trial product in Mexico
A multi-national sponsor needed a reliable provider with a wealth of expertise in delivering highly complex products from point of origin to patients at investigator sites, on time and in perfect condition.

Case study
Clinical supply optimization – mid-size pharma rejects bulk, avoids shortage
Many companies developing biosimilars are not confident in their own capacity to forecast supply needs and manage them on an ongoing basis.

Video
Advantages of Softgel Solutions for Developers
Kaspar van den Dries, Ph.D. discusses the many advantages for drug developers who choose Softgels.

Video
Bioprocessing Collaboration Center (BCC)
The new Bioprocessing Collaboration Center (BCC) in St. Louis, Missouri, uniquely positions Thermo Fisher to bring together industry-leading expertise in single-use technologies and biologics product development and manufacturing to deliver unmatched value to our customers.

Video
Benefits of Working with Patheon Softgels
Kaspar van den Dries, Ph.D. discusses the benefits of working with Patheon for your softgels project.

Presentation
How to pick the right CDMO for latephase clinical trials
For pharmaceutical companies without their own facilities, choosing the right partner for late phase clinical trials and commercial production is a critical consideration in developing parenteral products.

Presentation
Process characterization: Ready for the FDA?
As pipelines evolved to more complex molecules and manufacturing processes, though, the outlook of regulatory agencies on process characterization also changed.

Case study
Supplier collaboration delivers innovative solution for trial sponsor
In clinical trials using interactive response technology (IRT) drug dispensation is normally triggered after site activation or patient screening.

Case study
How clinical trial design impacts the supply chain
A pharmaceutical manufacturer established a head-to-head study comparing the safety and efficacy of Product A and Product B (the competitor’s product) for diabetic peripheral neuropathic pain (DPNP).

Case study
Clinical Ancillary Management, Solutions for Oncology Studies
Learn how to eliminate the need to determine unnecessary site-specific supplies and save months of work for all parties while increasing flexibility in the supply chain.

Whitepaper
ddPCR Quantification of Vector Genomes and Host Cell DNA
Learn more about the move toward using droplet digital polymerase chain reaction (ddPCR) in the quantification of AAV vector genome titer and residual host cell DNA.

Whitepaper
Anticipating the Formulation Challenges of Complex Molecules
Formulation problems are arising with greater frequency, delaying development, and burdening developers with unanticipated and heavy costs. Find out how to best anticipate formulation challenges of complex molecules and APIs.

Whitepaper
Are You Prepared for the Complexity of Pediatric Drug Development?
The numerous controls and processes in place to ensure a medication is safe, effective, and manufactured with the utmost efficiency make drug development extremely complicated.

Whitepaper
The Pharma Trends You Need to Know: A CDMO’s Perspective
The market momentum of novel therapies targeting unmet needs is creating a new landscape for pharmaceutical drug manufacturers. As the focus on these smaller patient pools grows, so does the complexity of drug development.

Whitepaper
Managing Demand Uncertainty in Biologics Production
For a small company for which liquidity is critical, tying up capital in a fallow facility due to improper forecasting can be catastrophic.

Whitepaper
Solutions to Today’s Biomanufacturing Challenges
Biopharmaceutical companies take on a lot of risk developing new large molecule drugs. With more complex molecules in development, changing capacity needs, uncertain forecasts and increased competition, the market demands flexibility and innovative approaches to address today’s new challenges.

Whitepaper
Consultants’ guide to flexible biomanufacturing solutions
Gain insight into key strategies that consultants can keep in mind for their smaller biopharmaceutical clients.

Whitepaper
Evaluating current manufacturing platforms for recombinant AAV production
Realizing the full potential of viral vector-based therapies requires a successful manufacturing platform for recombinant Adeno-associated virus (AAV) vectors.

Whitepaper
In-House Versus Outsource: A Decision-Making Guide
The biologics market is quickly evolving from a focus on developing blockbuster drugs to exploring niche markets with unmet needs.

Whitepaper
Technology Transfers: Reaping Rewards, Reducing Risks
In 2016, Patheon successfully completed 111 technology transfers to help our clients safeguard supply, improve distribution and reduce program costs and risks.

Whitepaper
Novel Uses for Oral Solid Doses Driving Lifecycle Management Strategies
With few potential blockbuster drugs in the pharma pipeline right now, drug companies are increasingly looking at other options to meet the needs of patients and increase revenue in the oral solid dosage (OSD) arena.

Whitepaper
Ensuring the greatest return from your poorly soluble molecule
The number of potential strategies for improving the solubility of a compound can overwhelm many developers.

Whitepaper
How to build a robust packaging strategy for rapid commercialization
When initial clinical trial results for a new drug show promise, the focus begins to shift to commercialization and planning for a pharmaceutical packaging strategy.

Whitepaper
5,000L single-use bioreactors: The next generation in biologics manufacturing
As the global biologics market and scientific advancements in biopharmaceuticals continues to rise, so has the demand for therapeutics, expanding indications for biologics, and the growing portfolios of biosimilars.

Whitepaper
Setting a strong foundation for your oral solid dose product to support late-stage development
Lengthy early development timelines are common across new & emerging biotech companies for a multitude of reasons.

Whitepaper
Sterile formulation strategies to shorten timelines for first-in-human studies
Lengthy early development timelines are common across new & emerging biotech companies for a multitude of reasons.

Whitepaper
Navigating the Adoption of Continuous Manufacturing Amid Unprecedented Global Challenges
Continuous manufacturing is an innovative solution, providing flexibility and up to 1.65X scale-up cost savings compared to batch manufacturing.

Whitepaper
Analytical Considerations For Biopharmaceuticals During Commercialization
To ensure the quality and safety of pharmaceutical drug products, it is critical to validate analytical methods before process performance qualification (PPQ).

Whitepaper
Who’s Watching Your Supply Chain? Strategies for Mitigating Risk in Clinical Trials
With the ever increasing costs of clinical trials and the advantages of being first-to-market, it’s time to take a closer look at your supply chain to ensure it delivers with speed, efficiency, and quality — all within your budget.

Whitepaper
Challenges and Practical Solutions for Switching to Prefilled Syringes for Injectables
Learn the critical patient benefits and improved outcomes of prefilled syringes, as well as how to select the most appropriate type and how to bridge vials with them.

Whitepaper
Product Development For An Oral Solid Dosage Using Continuous Manufacturing
When bringing a new drug to market, product development is typically aligned with the clinical trial schedule; therefore, as the patient pool grows, so does the supply of drugs needed for the study.

Whitepaper
The Right Partner Can Improve Flexibility and Mitigate Risks from Forecast Inaccuracy in Biomanufacturing
ORC International's report "Implications of Inaccurate Forecasting on Biologics Drug Substance Manufacturing" explores the causes, consequences, and potential solutions to forecasting challenges specifically related to biopharmaceutical drug substance manufacturing.

Whitepaper
The Race to Phase III: A Cautionary Tale of Scalability
When aggressive timelines are a “must,” it’s critical that companies don’t gloss over early-phase scale-up throughout the development process.

Whitepaper
Strategies for API Solubility and Bioavailability Enhancement
The landscape of today’s drug development industry looks far different than it did only a decade ago. Innovation is creating exciting new possibilities in patient care.

Whitepaper
Is Your In-House Strategy Ready for the Uncertainties of Biologic Drug Development?
The pharmaceutical industry’s past reliance on blockbuster drugs has evolved to include a focus on developing drugs that treat the unmet needs of smaller patient populations.

Whitepaper
Six API Challenges That Could Be Slowing Your Development and How to Avoid Them
Most APIs will require numerous steps or significant work upfront to avoid development delays, rework or outright failure.

Whitepaper
Rise in Targeted Therapies Drives Need for Small-Volume Manufacturing
Learn what pharma companies should look for when navigating this new era of small-volume manufacturing.

Whitepaper
Quality by Design: A Holistic Approach to Drug Development
Learn how sponsors who implement QbD early can save money through increased product / process knowledge, less re-work, less product deviation, less product out-of-specification, fewer rejects and improved quality.

Whitepaper
Impact of Incorrect Forecasts on New Product Launches
Pharmaceutical companies around the world are under tremendous pressure – from regulators, legislators, payers, and patients – to reduce the cost of drugs.

Whitepaper
How Well Should You Understand Your Crystallization Process (and Solid-State Properties)?
Crystallization is the most common unit operation for isolation and purification of solid intermediates and active pharmaceutical ingredients (APIs).

Whitepaper
Getting to First-in-Human Clinical Trials: A Make-or-Break Milestone for Small Biopharmas
"Faster and better" has become the mantra for biopharmaceutical companies as they face intense pressure to get therapies to market quicker than ever before.

Whitepaper
Decision Timeline: Considerations in Selecting an Outsourced Solution
The traditional business model for in-house pharmaceutical manufacturing is nearly a thing of the past. More companies are turning to outsourcing to achieve flexibility and efficiency in a highly competitive market.

Whitepaper
Create Brand Sustainability with Softgel Technology
Learn how innovation, particularly around softgels, is key to gaining consumer loyalty and extending a product’s lifecycle.

Whitepaper
Choosing the Best Sterile Dosage Form for Your Phase I Clinical Supply Needs
There are many parenteral dosage forms from which the pharmaceutical scientist can choose to develop their drug product.

Whitepaper
Challenges and Solutions in Cytotoxins and HPAPIs
We live in a time when breakthrough medicines are being discovered at an unprecedented rate. Yet whether from big pharmaceutical companies or small hubs of innovation, these treatments of tomorrow are often held up by a complex supply chain. Patheon offers a cure.

Whitepaper
Built To Fail: How Today’s Manufacturing Options Leave Pharma At Risk
When planning for the commercialization of a new product, pharmaceutical manufacturing executives must plan for capacity needs very early in the process.

Whitepaper
Challenges and Practical Solutions for Switching to Prefilled Syringes for Injectables
Learn the critical patient benefits and improved outcomes of prefilled syringes, as well as how to select the most appropriate type and how to bridge vials with them.

Whitepaper
Comparator Local Sourcing Strategies
Learn about alternative local sourcing strategies and get information on the advantages and disadvantages of local sourcing while remaining compliant.

Whitepaper
Comparator local sourcing strategies: Leveraging advantages and avoiding common pitfalls for clinical trials
This whitepaper will focus on local sourcing. Local sourcing is the purchase of a commercial drug within a single country for use in that same country.

Whitepaper
5 Big Common Missteps in Comparator Sourcing
Do you know the 5 big common missteps in comparator sourcing? Download our infographic to find out.

Whitepaper
Small molecule orphan drugs: Balancing financial incentives and complex challenges
For companies pursuing an orphan drug, our experts help guide you through the complex challenges of getting your small molecule orphan drug to market faster.

Webinar
Accelerating Cell Culture Development through Practical Application of Innovation Technologies
Changes in the biologics pipeline as well as changes in commercialization needs trigger changes in the technologies we use and work flows for cell line development.

Webinar
Accelerating cell line and cell culture development through practical application of innovative technologies
Speaker: Palak Patel, Scientist, Cell Culture Development
– The global biologics pipeline continues to fuel “speed-to-clinic”

Webinar
Accelerating innovative therapies to patients in a post pandemic world
Learn from industry experts and esteemed guests on hot topics including:
From research to clinic: the path to success for cell therapy companies
Evaluating options for outsourcing discovery research

Webinar
Accelerate late-phase drug development with continuous manufacturing
The process development of oral solid dosage forms occurs across multiple phases. The final scaled up process is not typically finalized until shortly before filing for commercial approval.

Whitepaper
What you need to know about process characterization and validation for biologic processes
A major factor in the growth of the biopharmaceutical industry over the last 20 years has been continuous innovation with monoclonal antibodies (mAbs), which now make up more than 50% of the overall biotherapeutic market.

Whitepaper
Top five risks facing your small biopharma clients
Biopharma firms function in a risky development environment with compressed timelines and budget constraints. Companies often overlook critical factors which could delay or suspend efforts down the road. Consultants should help clients understand five key risks in order to avoid costly problems and maximize financial returns during the development process.

Whitepaper
Synthesizing success: Six principles for getting pharmaceutical development right from the start
Today’s market demands speed, flexibility, and innovation to address today’s new challenges. As a result, pharmaceutical and biotechnology companies face increasing pressure to bring blockbuster drugs to market quickly and cost-effectively.

Whitepaper
Critical API attributes and the major impact they can have on drug product development
Discover why an alternative, integrated approach to formulation should be considered based on the impact of certain API properties on the final drug product.

Whitepaper
Flow chemistry: A scaleup solution for modern API development & manufacturing
Once deemed an “experts-only” approach to chemical synthesis, flow chemistry is a cost-efficient technique growing in popularity that can increase safety and flexibility and improve product quality.

Whitepaper
Accelerate complex molecule development by optimizing chemical synthesis and formulation
Innovations in science and technology over the last few decades enable scientists to create far more advanced pharmaceuticals for today’s industry.

Whitepaper
What you need to know to avoid costly delays in your API scale-up
Traditionally, the development of a small-scale synthesis for an active pharmaceutical ingredient (API) and its scale-up to meet the materials demand for clinical trial phases is a sequential activity that passes through multiple sets of hands.

Whitepaper
Challenges, risks, and strategies for biologic drug substance manufacturing
Insights from pharmaceutical and biotechnology industry leaders make it clear that demand forecasting is a significant challenge when planning biologic drug substance production.

Whitepaper
How can you avoid the fallout from incompatibility between your API and its formulation?
In drug development, designing a formulation for a drug product (a tablet, for example) calls for careful attention to both the physical and chemical properties of the active pharmaceutical ingredient (API or drug substance).

Whitepaper
Impact of a pandemic outbreak on vaccine development approach
Vaccine development has its own set of rising complex challenges. However, when it comes to vaccine development during a pandemic, the response has its own set of complex challenges that traditional manufacturing methods won’t solve for.

Whitepaper
Implications of inaccurate forecasting on biologics drug substance manufacturing
An Independent Executive Research Study by ORC International examines how large molecule drug substance manufacturing and demand forecasting is riddled with complexity.

Whitepaper
Multiplexing: Managing risk with proven, single use solutions
When pharmaceutical companies introduce a new drug to market, they invest enormous amounts of capital, and assume
equally enormous amounts of risk.

Whitepaper
Bulletproof your supply chain: Hope for the best, prepare for the worst
Hope is not a strategy in drug manufacturing. You need a contingency plan to protect your company and your customers if the worst-case scenario occurs.

Whitepaper
What clinical teams should know about changing trial logistics and how they will affect development
When it comes to clinical supplies, the journey is every bit as important as the destination. And these days, the journey of clinical supplies to investigator sites is becoming costlier and more complex, much like the global trials for which the materials are bound.

Whitepaper
Comparator local sourcing for clinical trials: Balancing opportunities and challenges
There are several reasons why the market for comparators is growing. For one thing, more clinical trials are being conducted today than ever before and more of those trials are using active comparators.

Whitepaper
Opportunities and challenges for clinical research in China
Today China is a dominant presence in the clinical trial arena and one of the most desirable

Whitepaper
Continuous or batch: Deciding on the best solution for your oral solid dose product
Overview the advantages of continuous manufacturing for your oral solid dose product.

Whitepaper
Hot melt extrusion: Improving solubility of poorly soluble compounds
Solving solubility challenges before they become long-term issues is critical for the success of your small molecule project.

Whitepaper
Hot melt extrusion: Improving solubility of poorly soluble compounds
Solving solubility challenges before they become long-term issues is critical for the success of your small molecule project.

Whitepaper
Manufacturing Process Scale-up for Phase III: Clear Sailing or Storms Ahead?
Learn how building scalability into formulation throughout drug development, as well as transferring the project to a knowledgeable CDMO in Phase III, can help mitigate some of the challenges that arise in Phase III.

Whitepaper
How Broadening the Analysis of Compound Factors Allows for Predictive Solubility Solutions
The Biopharmaceutics Classification System (BCS), developed by the U.S. Food and Drug Administration to simplify and accelerate the drug development process, helps companies when they file for bioequivalence of dosage forms based on in vitro dissolution testing.

Fact sheet
CMO Scorecard
Find out how we helped clients earn as many NDA approvals as the next three leading CMOs combined.

Fact sheet
CGT commercial services capabilities
Discover how Patheon leverages 35+ years of experience and a global network to support the secondary packaging, labeling, and distribution of your life-saving cell or gene therapy.

mRNA manufacturing services overview
The emergence of mRNA therapeutics including the development of new vaccines and gene therapies has created a market constraint on access to critical raw materials and technical expertise.

Fact sheet
Qualified Person (QP) Services
Learn how our team of QPs bring extensive experience across a wide range of dosage forms including aerosols, biologicals, creams, liquids, ointments, solids, sterile products and novel drug delivery systems.

Fact sheet
CTX Solutions Fact Sheet
The cell and gene therapy market is experiencing accelerated market approval opportunities, record breaking investments, robust therapeutic pipelines, and positive clinical outcomes – all driving the need for speed, regulatory know-how, and innovation in manufacturing technologies.

Fact sheet
Patheon™️ Quick to Clinic™️ Viral Vector Services
Patheon™️ Quick to Clinic™️ Viral Vector Services Patheon™️ Quick to Clinic™️ viral vector services is an integrated development program utilizing... Read More

Fact sheet
Engineered Solutions for oral solid dose product development
Engineered solutions adopt a comprehensive view of drug product development by looking at pharmaceutical ingredients, related manufacturing processes, products, and biopharmaceutical properties and how they are intrinsically connected and critical to the success of your product development.

Location
Five reasons to consider softgels as a new over-the-counter dose form to improve market share
As the drug product market becomes increasingly competitive, new dose formats for over-the-counter (OTC) drugs is critical to maintaining and growing market share. We’ve rounded up five common scenarios when a softgel format can increase benefits for consumers and improve business results.

Infographic
5 Common Clinical Supply Chain Speedbumps and How the Right Partner Can Help Avoid Them
Download this infographic to learn about 5 common issues, and why it is important to select the right partner.

Infographic
Depot to Patient Service Overview
Download this infographic to learn more about the Depot-to-Patient service.

Infographic
10 Ways to Derail a Clinical Trial
Learn important factors to consider when developing your clinical supply planning strategy to avoid derailing your clinical trial.

Whitepaper
EU Clinical Trial Regulation 2022: Understanding the impact on clinical research in Europe
Learn more about key changes introduced by the EU Clinical Trial Regulation (CTR) 2022 regulation, with guidance for managing anticipated challenges.

Whitepaper
Continuous manufacturing and late-phase strategy: The time is now
Continuous manufacturing is revolutionizing the pharmaceutical industry. This seamless manufacturing process reduces overall time to produce a drug product from days and weeks with batch manufacturing to mere hours.

Whitepaper
Patient-centric oral solid dose formulation: Improving access and value across the product lifecycle
Learn how focusing on your patient during the drug development process can lead to improved treatment adherence, clinical outcomes, and market access.

Whitepaper
Rising to the rare disease challenge: Key considerations in large-molecule orphan drug development
Learn how to overcome the challenges within drug development due to small-scale manufacturing, limited time/data, small sample sizes, and costs.

Article
Debunking Myths and Misconceptions About Softgels
Explore seven myths about softgel formulation for prescription drugs and why this dosage form is more viable than formulators may assume.

Article
Using softgel innovations to grow market share
Softgel capsules are one example of a highly versatile dose form that can improve consumers’ experience of your drug product and create growth opportunities for your business.

Article
Ancillary supplies clinical trial must haves that require early planning
Learn why ancillaries are as essential as IMPs, and why sponsors should devote as much attention to early planning for ancillary supplies as they do for study drugs.
Article
A comprehensive approach to improving solubility and bioavailability Spray drying
Spray drying is a solution that is applicable to a broad range of API’s. Read more about the critical components to spray drying in manufacturing & more.

Infographic
5 Big Common Missteps in Comparator Sourcing
Do you know the 5 big common missteps in comparator sourcing? Download our infographic to find out.

Infographic
8 Criteria for a More Successful API Partnership
Outsourcing API development can save time and money. Or it can waste them. Since the difference between these outcomes stems from your choice of a development partner, careful consideration of these eight critical areas is required to help ensure a fast, smooth API development process.

Infographic
Direct to Patient Pharmacy Services
Download this infographic to learn more about the supply chain options for decentralized clinical trials.

Infographic
Five business advantages of softgels for over-the-counter products
Considering a new dose format can be a strategic lever for your over-the-counter portfolio. Softgels as that dose format can support an increase in patient compliance, consumer preference, and grow your market share. Uncover the business advantages a softgel format can deliver to your business with this infographic.

Infographic
Four Ways to Accelerate Downstream Development
Complete investigation of your API is a critical step in the development process. When investigations are pressured by the delicate balance of time and budget, formulation decisions can be made that impact downstream processes.

Infographic
Rethink common myths and misconceptions about softgels as an optimal dose format
Softgels are widely used today across prescription and over-the-counter formulations due to their patient friendly format. Read more about common misconceptions related to softgels and how to debunk these myths within your business to capture more market share.

Infographic

Webinar
Leveraging Innovative Technologies, Best Practices and Strategies to Accelerate Biologics Development and Commercialization
Gain insight into how practical implementation of innovative technologies and solutions help to accelerate biologics development and commercialization.

Whitepaper
Factoring the “what ifs” into supply forecasting: Why building a durable supply chain around a protocol is critical
Discover challenges and aspects to consider when developing a supply plan, the influence of early decisions and impact on outcome as a trial progresses, and how decisions can put patients and the trial at risk.

Whitepaper
Fixed-dose combination drugs: Innovative formulation and development strategies for bringing best-in-class products to market
Learn how our tools can accurately identify effective combinations for many drugs on the market and help avoid those that may be problematic.

Infographic
10 Steps That Risk Failure in the Temperature Controlled Clinical Supply Chain
Download this infographic to understand the questions you should be asking to be better positioned to ensure zero excursions and maximum product integrity.

Whitepaper
Biologic drug products: A 5-point strategy for building a robust CMC dossier
Explore five strategies for building a robust CMC package to help streamline the path to FIH clinical trials for biologics.
Infographic
Maintaining the Cold Chain in European Distribution
Learn top tips for maintaining cold chain integrity across the European supply chain with the growth of biologics and vaccines in the biopharma sector.

Infographic
Made with proof & purpose
Made with proof & purpose Your molecule has the potential to change lives and shape the future.

Whitepaper
Avoid the do-over: Why early investment in a scalable manufacturing process is critical
The logistical challenges of matching drug supply to research needs in clinical trials have increased by an order of magnitude in recent years.

Whitepaper
Clinical trial simulation: Advanced modeling techniques enable data-driven supply chain planning
The logistical challenges of matching drug supply to research needs in clinical trials have increased by an order of magnitude in recent years.

Whitepaper
Characterizing drug substance properties early can optimize drug product formulation
Changes in the Drug Substance (DS) process as it scales up can affect the Drug Product (DP). As processes change, many properties of the DS can also change.

Whitepaper
Enabling fast and appropriate drug product supply for phase 1 clinical trials
Reducing the timeline from conception to Phase 1 trials can be especially challenging for new and emerging biotechs.

Whitepaper
Solving the OOS problem with continuous manufacturing
The goal of Pharmaceutical Process design has long been to implement a fixed process that can produce on-spec materials, which can be confirmed by quality testing.

Whitepaper
Clinical Trial Supply Solutions
Whether you need primary or secondary packaging of your clinical drug, storage, distribution, logistics, cold chain management, or comparator or ancillary sourcing, our global team can meet the needs of every trial regardless of size, phase or therapeutic area.

Whitepaper
Full throttle for vaccine filling
The recent COVID-19 pandemic placed unique demands and challenges on vaccine producers, equipment manufacturers and distributors.

Whitepaper
Telltale signs you’re with the wrong CDMO
Late-stage drug development efforts have many moving parts as projects push toward commercialization.

Whitepaper
Understanding the CMC regulatory landscape for cell and gene therapy products
Like all biotech products, getting life-saving medicines to market quickly can be delayed if you’re not prepared for regulatory inspections.

Whitepaper
Addressing the complexity of process validation for cell and gene therapy products
Learn how to develop a robust validation package that supports cell and gene therapies.

Whitepaper
Strategic CDMO partnerships: Leveraging infrastructure investments and innovation to accelerate biologics development
Rising to the challenges of biologics development in this period of disruptive change requires an appreciation for the science, technology, and market forces driving the transformation and a willingness to adopt strategies that align with these changes.

Whitepaper
Comparator local sourcing strategies: Leveraging advantages and avoiding common pitfalls for clinical trials
This whitepaper will focus on local sourcing. Local sourcing is the purchase of a commercial drug within a single country for use in that same country.

Whitepaper
What every clinical team should consider before developing the next protocol: Putting volunteers first
Learn why even though everyone applauds the renewed focus on patients as a positive development, some say it is time for biopharma companies to turn their attention to another audience.

Whitepaper
Considerations for your first clinical trial
Learn why sponsors should consider partnering with an organization with demonstrated expertise, ability to work across geographies and cultures, a keen understanding of local and international requirements, and that offer models for support at multiple levels.

Whitepaper
Considering the clinical supply chain in vaccine trials: Special handling required
Vaccines are one of the most useful and cost-effective means of reducing illness and death from infectious diseases. With hundreds of vaccines in research and development worldwide, vaccines are among the fastest growing segments of the biopharmaceutical market today.

Whitepaper
How explosive growth in biosimilars presents new challenges in the clinical trial supply chain
With patent protection for many innovator biological drugs expiring within the next few years, the early 21st century may well be remembered as the dawn of the Biosimilar Era, and for good reason.

Whitepaper
Focus on drug delivery What clinical teams should know about the benefits of auto-injectors
More drugs are being delivered by injection than ever before, fueled by a steady stream of biological products emerging from research pipelines.

Whitepaper
Cold chain fully automated assembly and labeling of pre-filled syringes for clinical trials
Through 2022, and in the future, we’ll continue to see significant growth in the sale of cold-chain drugs.

Whitepaper
Managing supply logistics in an expanding clinical trial universe: New challenges for global clinical trials
Today, half of all clinical trials are conducted offshore and in more developing countries than ever before, profoundly increasing the complexity of supply chain logistics.

Whitepaper
Ukraine & Russia: Opportunities and Challenges for Clinical Research
Discover why Ukraine and Russia are among the top choices for clinical development.

Blog post
The Four Stages of Equipment Qualification
As discussed in my previous blog, qualification is the process of establishing documented evidence that a specific equipment, facility or system are fit and ready for their intended use.
3 minute read

Blog post
Myths & Facts about Ancillaries
It’s a fact that ancillary supplies are frequently perceived as less important than study drug.
2 minute read

Blog post
Is Your Supply Chain Bulletproof?
Before you respond, think hurricanes, Nor’easters and tsunamis. Earthquakes, typhoons and volcanic eruptions. Civil unrest and war. Terrorism. A pandemic virus.
2 minute read

Blog post
Top Tips on How to Manage Clinical Label Translation and Regulatory Requirements
Wouldn’t it be nice to find a way to stay on top of label translation and regulatory requirements for every country included in your clinical trial?
2 minute read

Blog post
Introducing an Expanded Packaging Service for Specialty Products
Developing a specialty drug for a complex or rare disease is an achievement worthy of celebration.
2 minute read

Blog post
Is it Time to Start Thinking about Packaging?
If this headline caught your eye, it may be because you’ve received promising pre-clinical results (Congratulations!) and you’re starting to think about planning your next steps.
2 minute read

Blog post
Taking Your API to the Next Level: Three Steps to Consider Before Outsourcing
With outsourcing API development becoming more common, we see a rise of multiple competing Contract Development Manufacturing Organizations (CDMOs) as potential development partners.
10 minute read

Blog post
Break Through the OTC Noise
It’s no secret—consumers have a vast range of OTC options at their favorite in-store or online retailer. Everything from tablets and capsules, to syrups—consumers have more options than ever in the OTC jungle.
8 minute read

Blog post
Considerations and roadblocks that stifle orphan drug development
According to the US Food and Drug Administration (FDA), “2020 was a record-breaking year in terms of the number of orphan drug designation and rare pediatric disease designation requests submitted to the Office of Orphan Products Development.”
6 minute read

Blog post
Regulatory landscape in Europe: Key advice for meeting post-Brexit Qualified Person requirements
The United Kingdom’s (UK) departure from the European Union (EU) has added a layer of complexity to the clinical trial supply chain in Europe above and beyond COVID-related disruptions.
(4 minute read)

Blog post
Time to embrace electronic labels? Potential labeling solutions under the new EU Clinical Trial Regulation
The new EU Clinical Trial Regulation (CTR) is intended to simplify clinical trial administration and create a more welcoming climate for pharmaceutical companies that operate in Europe.
5 minute read

Blog post
Navigating the Complexities of Process Performance Qualification
Method qualification is monumentally important before process performance qualification (PPQ). This early assessment of your method’s performance characteristics is critical as it pertains to method validation and its parameters such as precision, accuracy, and linearity.
5 minute read

Blog post
Continuous Manufacturing: An Efficient Way to Produce OSD Drugs
When Henry Ford revolutionized manufacturing practices back in 1913 in Highland Park, MI, his main goal was simple—to make the best possible product in the most efficient and cost-effective manner. Ford’s focus on bettering the “flow” of manufacturing to enable workers/technology to work smarter and reduce waste of raw materials, changed manufacturing principles forever.
6 minute read

Blog post
Simplifying Your API's Development Journey
The primary way new drugs are developed is changing. Historically, pharma companies—particularly large pharma companies—have developed APIs in-house.
7 minute read

Blog post
COVID-19’s Silver Lining: Accelerated Vaccine Development
Vaccine development is a lengthy process—it is expensive, attrition is high, and to get a licensed vaccine to everyone, it takes multiple candidate iterations. Vaccine development for pandemics and epidemics is risky, and due to the novel nature of viruses, certain unknown factors can derail a vaccine program.
6 minute read

Blog post
A Day in the Life of a Viral Vector Partner
When it comes to a viral vector Contract and Development Manufacturing Organization (CDMO), what sort of qualities should they possess?
9 minute read

Blog post
How Decentralized Clinical Trials Enhance Patient-Centricity in the Age of COVID-19
As COVID-19 continues to change how we do business in the biopharmaceutical industry, it’s important to not lose sight of why we do what we do: improving and saving patient lives.
5 minute read

Blog post
Work Smarter, Not Harder: Accelerating Your Biologics Development and Commercialization
Over the past decade, the biologics industry has seen double digit growth and an overall increase of market share.
5 minute read

Blog post
Prefilled Syringes: Three Pain Points You May Not Have Considered
As pharmaceutical companies look to become more patient-centric, certain drug products come to the forefront to support that effort.
5 minute read

Blog post
Navigating Cell & Gene Therapy Regulations: How Does Your CDMO Match Up?
Whether you are a large or new and emerging biotech company, many companies find themselves lacking the internal resources and/or expertise to properly support regulatory submissions.
7 minute read

Fact sheet
Comparator and Co-Medication Sourcing Services
Learn how our Comparator Center of Excellence Team can assist you with developing the most cost effective strategy for securing the medications locally, regionally, globally or a combination of all the above.

Article
Streamlining small molecule API development and regulatory compliance
The end goal of API innovators is to develop an API that will succeed in clinical trials. Many steps and processes are considered in formulation and development that would enable an API to successfully transition to the clinic.

Interview
Ensure a timely arrival
Stalled deliveries of clinical supplies to global investigator sites have the potential to derail trials and put study patients at risk. Clinical Trial Insight talks to Ian Hunter, commercial director of logistics at Fisher Clinical Services, about the company’s strategy for minimizing delays to ensure clinical supplies reach the sites and patients who need them on time.

Blog post
Choosing a CDMO Who is a True Bioproduction Expert
With the number of CDMO’s rising in the biologics manufacturing industry, it can be challenging for new and emerging biopharmaceutical companies to determine which CDMO is right for them.
8 minute read

Fact sheet
Cold Chain Management & Mindset
Learn how our integrated processes, the latest technology, and our zero temperature excursion mindset mitigates the risk of temperature excursion throughout the end-to-end supply chain.

Fact sheet
Clinical Supply Optimization Services
Learn how Clinical Supply Optimization Services can optimize and streamline the clinical supply chain from early strategy development through packaging, distribution, site inventory management, and returns/destruction.

Fact sheet
Clinical Ancillary Management
Learn how a highly organized and rigorously managed process can increase study efficiency and minimize the risk of your patient missing a dose.

Fact sheet
ATLAS (Alternative Translation and Label Approval System)
Learn about our proprietary, web-based Alternative Translation and Label Approval System (ATLASSM)—a validated, clinical label translation management service that automates and optimizes multi-lingual label text development.

Fact sheet
Global Site Network: End-to-End Pharma Services
Learn more about our fully integrated global site network that can support your molecule from development to commercialization.

Fact sheet
Patheon pharma services: Your truly integrated partner for drug development
Advance your discovery from molecule to medicine with our industry-leading, end-to-end pharma services capabilities to simplify your supply chain. Learn more about how we can partner with you on your drug development journey.

Fact sheet
Global steriles investment & innovation
The medicines we help develop and deliver for our customers impact one million patients each day. With an increasing demand for our services, expertise and capabilities, comes a commitment to continuously expand our products and services. This is why we have been making unprecedented investments in our network of sites and capabilities around the world. Learn more about our investments in Steriles within North America, Asia-Pacific, and Europe.

Fact sheet
mysupply Platform: An End-to-End Digital Supply Chain Platform
From development to launch, pharma products navigate a dynamic global supply chain with potential changes and delays

Fact sheet
Global Label Services
Learn how our Global Label Services team leverages purpose built, integrated facilities, a global presence and the information systems and flexibility to allow unparalleled visibility and control while offering time-saving, seamless transition opportunities throughout the clinical supply chain.

Fact sheet
Global Logistics Help Desk
Learn how data intelligence is used to select the best courier for each country, helping our Sponsors to realize cost and performance efficiencies across the supply chain while mitigating risk and adhering to clinical trial timelines.

Fact sheet
Pre-Filled Syringe Assembly & Labeling
Learn about our fully automated, continuous process from syringe assembly to label application to electronic verification of label text.

Fact sheet
Reusable Shipper Program
Learn how a reusable shipper program can bring multiple benefits for all stakeholders and further increases the quality across the supply chain.

Fact sheet
Investigator Initiated Trial (IIT) Service for Institutions
Learn how our IIT service offers comprehensive oversight and management of all clinical supply activities for a portfolio of Investigator Initiated Trials.

Fact sheet
Investigator Initiated Trial (IIT) Service for Pharma Companies
Learn how our IIT service offers comprehensive oversight and management of all clinical supply activities for a portfolio of Investigator Initiated Trials.

Fact sheet
Total Transportation Management for the Life Science Industry
Learn how our Total Transportation Management service helps sponsors manage the complex supply chain processes required to move manufacturing API, IMP, comparator medication and ancillaries through to commercial goods, both internationally and within country of destination.

Fact sheet
Direct-to-Patient Services
Download this fact sheet to learn about end-to-end solutions that connect your treatments directly with your patients.

Fact sheet
Total Transportation Management for the Life Sciences Industry
Learn how a comprehensive transportation management strategy can help you access a global network of couriers, understand shipment delays and save at-risk shipments.

Fact sheet
Quick to Clinic<sup>TM</sup> for Oral Solid Dose
Only 12 weeks from receipt of your API, Patheon Quick to Clinic™ can deliver bulk clinical trial materials for first-in-human studies. Learn how.

Fact sheet
Sample Fulfillment & Distribution Overview
Learn more about our full range of services in support of your Direct-to-Representative and Direct-to-Practitioner sampling programs.

Fact sheet
API (Small Molecule) Overview
Find out what it means to take a big picture approach to small molecule development to deliver high-quality API via a seamlessly scalable process that will rapidly achieve your goals at each phase of development while laying a sound foundation for your future success.

Fact sheet
Biologics Services Overview
Learn more about Thermo Fisher Scientific’s industry-leading expertise and CDMO services for biologics development and manufacturing.

Fact sheet
Early Development Fact Sheet
Discover how Patheon can provide you with customized early development strategies and technical solutions to solve complex development challenges.

Fact sheet
OnTheGo℠ Suite of Services
Discover how Patheon Logistics’ PDMA-compliant suite of OnTheGo mobile and web apps optimize sales representative productivity and enhance practitioner convenience and service.

Fact sheet
Softgel Technologies
Learn more about our softgel offerings and how to leverage our broad range of expertise, from pre-formulation through commercialization, to help your project achieve success.

Fact sheet
Specialty Courier Services Overview
Learn more about our ability to design an optimal specialty courier solution that includes everything from recommended shippers to facilitating import/export clearance.

Fact sheet
Preferred Carrier Management Overview
Learn how our Life Sciences Standards shipments are restricted to a Preferred Carrier network which is uniquely specialized to include temperature controlled and validated trailers.

Fact sheet
Continuous Manufacturing: The Alternative to Batch Manufacturing
Discover how continuous manufacturing can deliver higher quality oral solid dose products, with greater flexibility and a reduced total cost of supply.

Fact sheet
Commercial Packaging Overview
Learn about our flexible integrated end-to-end solutions and technical expertise in clinical and commercial packaging combined with other value-added services.

Fact sheet
Viral Vector Services Regulatory Offering
Learn how Patheon Viral Vector Services, with over 15 years’ experience, provides a comprehensive range of regulatory consulting services for the cell and gene therapy innovators.

Fact sheet
Fill/Finish Services for Viral Vectors
Learn more about our flexible, comprehensive Fill/Finish services for viral vectors that comply with current regulatory and quality requirements.

Fact sheet
Quadrant2 Predictive Platform for Solubility and Bioavailability Enhancement
Quadrant 2® is a diagnostic tool for early development, which allows us to see in-silico predictions of formulations. The results of this diagnostic tool can save time and costs by avoiding trial and error approaches because we can quickly provide a solution to enhance solubility.
Poster
A Quality by Design Approach to Optimize Tablet Formulations Containing PVP VA64 Amorphous Dispersions: Formulation Variables

Biotech solutions: Turn breakthrough ideas into real-world patient impact
From early development through commercialization, our CRO experts help you speed up timelines, elevate outcomes, and simplify complexity—providing support at every stage of the journey.

On-demand webinar: Speeding up clinical trial timelines with Quality by Design
In clinical research, speed and quality are often seen as mutually exclusive, but that’s not always accurate. A Quality by Design approach can help you run faster, more efficient trials.

On-demand webinar: Intelligent drug development for small and emerging sponsors
Small and emerging biopharma companies often face a fragmented development landscape on the way to Phase II. Learn how a more connected model can position your program for success.

Video
Advantages of Softgel Technologies with Tony van Bijleveld
Tony van Bijleveld, Vice President, Sales, discusses the benefits of the recent Thermo Fisher Scientific acquisition, as well as Softgel expertise & technology to enable innovative customer solutions for patients.

